Key Points
-
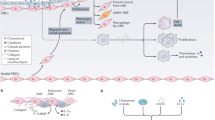
Atherosclerosis is a chronic inflammatory disease of the arterial walls and is the primary cause of cardiovascular disease
-
Statin therapy is not effective in reducing cholesterol levels in a small proportion of users and prolonged use of statins can increase the risk of adverse effects
-
Nutraceuticals are natural compounds derived from food sources that are known to be beneficial against disease
-
Several nutraceuticals have been shown to have anti-inflammatory and antioxidative properties, and are therefore promising compounds to explore as novel antiatherogenic preventive therapies
-
Although some nutraceuticals have shown promise in observational studies, large, robust clinical trials are required to determine their efficacy in attenuating atherosclerotic disease progression
Abstract
Atherosclerosis is a chronic inflammatory disease affecting large and medium arteries and is considered to be a major underlying cause of cardiovascular disease (CVD). Although the development of pharmacotherapies to treat CVD has contributed to a decline in cardiac mortality in the past few decades, CVD is estimated to be the cause of one-third of deaths globally. Nutraceuticals are natural nutritional compounds that are beneficial for the prevention or treatment of disease and, therefore, are a possible therapeutic avenue for the treatment of atherosclerosis. The purpose of this Review is to highlight potential nutraceuticals for use as antiatherogenic therapies with evidence from in vitro and in vivo studies. Furthermore, the current evidence from observational and randomized clinical studies into the role of nutraceuticals in preventing atherosclerosis in humans will also be discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Health Organization. Cardiovascular diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en/ (2015).
McLaren, J. E., Michael, D. R., Ashlin, T. G. & Ramji, D. P. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog. Lipid Res. 50, 331–347 (2011).
Vogel, R. A. Coronary risk factors, endothelial function, and atherosclerosis: a review. Clin. Cardiol. 20, 426–432 (1997).
Ramji, D. P. & Davies, T. S. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 26, 673–685 (2015).
Buckley, M. L. & Ramji, D. P. The influence of dysfunctional signaling and lipid homeostasis in mediating the inflammatory responses during atherosclerosis. Biochim. Biophys. Acta 1852, 1498–1510 (2015).
Chistiakov, D. A., Bobryshev, Y. V. & Orekhov, A. N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 20, 17–28 (2016).
McLaren, J. E. & Ramji, D. P. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 20, 125–135 (2009).
Moss, J. W. E. & Ramji, D. P. Interferon-γ: promising therapeutic target in atherosclerosis. World J. Exp. Med. 5, 154–159 (2015).
Chistiakov, D. A., Orekhov, A. N. & Bobryshev, Y. V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 214, 33–50 (2015).
Katsuda, S. & Kaji, T. Atherosclerosis and extracellular matrix. J. Atheroscler. Thromb. 10, 267–274 (2003).
Newby, A. Matrix metallproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 69, 614–624 (2006).
Haslinger-Loffler, B. Multiple effects of HMG-CoA reductase inhibitors (statins) besides their lipid-lowering function. Kidney Int. 74, 553–555 (2008).
Leitersdorf, E. Cholesterol absorption inhibition: filling an unmet need in lipid-lowering management. Eur. Heart J. Suppl. 3, E17–E23 (2001).
Parker, B. A. et al. Effect of statins on skeletal muscle function. Circulation 127, 96–103 (2013).
Calderon, R. M., Cubeddu, L. X., Goldberg, R. B. & Schiff, E. R. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin. Proc. 85, 349–356 (2010).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Tsujita, K. et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention the multicenter randomized controlled PRECISE-IVUS trial. J. Am. Coll. Cardiol. 66, 495–507 (2015).
Patel, A. Y., Pillarisetti, J., Marr, J. & Vacek, J. L. Ezetimibe in combination with a statin does not reduce all-cause mortality. J. Clin. Med. Res. 5, 275–280 (2013).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01764633 (2016).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01663402 (2016).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01975376 (2016).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/results/NCT01975389 (2016).
Ridker, P. M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ. Res. 118, 145–156 (2016).
Slavin, J. L. & Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 3, 506–516 (2012).
Wall, R., Ross, R. P., Fitzgerald, G. F. & Stanton, C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 68, 280–289 (2010).
Granados-Principal, S., Quiles, J. L., Ramirez-Tortosa, C. L., Sanchez-Rovira, P. & Ramirez-Tortosa, M. C. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 68, 191–206 (2010).
Lee, J. H., O'Keefe, J. H., Lavie, C. J., Marchioli, R. & Harris, W. S. Omega-3 fatty acids for cardioprotection. Mayo Clin. Proc. 83, 324–332 (2008).
Lavie, C. J., Milani, R. V., Mehra, M. R. & Ventura, H. O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54, 585–594 (2009).
American Heart Association. Fish and omega-3 fatty acids. http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/HealthyDietGoals/Fish-and-Omega-3-Fatty-Acids_UCM_303248_Article.jsp# (2015).
Bang, H. & Dyerberg, J. Lipid metabolism and ischemic heart disease in Greenland Eskimos. Adv. Food Nutr. Res. 3, 1–22 (1980).
Sanders, T. A. Polyunsaturated fatty acids in the food chain in Europe. Am. J. Clin. Nutr. 71, 176S–178S (2000).
Simopoulos, A. P. Importance of the ratio of omega- 6/omega-3 essential fatty acids: evolutionary aspects. World Rev. Nutr. Diet 92, 1–22 (2003).
James, M. J., Gibson, R. A. & Cleland, L. G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 71, 343S–348S (2000).
Tsimikas, S. et al. LDL isolated from Greek subjects on a typical diet or from American subjects on an oleate-supplemented diet induces less monocyte chemotaxis and adhesion when exposed to oxidative stress. Arterioscler. Thromb. Vasc. Biol. 19, 122–130 (1999).
Das, U. N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 7, 37 (2008).
Hughes, D. A., Southon, S. & Pinder, A. C. (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J. Nutr. 126, 603–610 (1996).
Miles, E. A., Wallace, F. A. & Calder, P. C. Dietary fish oil reduces intercellular adhesion molecule 1 and scavenger receptor expression on murine macrophages. Atherosclerosis 152, 43–50 (2000).
Brown, A. L. et al. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler. Thromb. Vasc. Biol. 32, 2122–2130 (2012).
Song, Y. et al. Polyunsaturated fatty acid relatively decreases cholesterol content in THP-1 macrophage-derived foam cell: partly correlates with expression profile of CIDE and PAT members. Lipids Health Dis. 12, 111 (2013).
McLaren, J. E., Michael, D. R., Guschina, I. A., Harwood, J. L. & Ramji, D. P. Eicosapentaenoic acid and docosahexaenoic acid regulate modified LDL uptake and macropinocytosis in human macrophages. Lipids 46, 1053–1061 (2011).
Lada, A. T., Rudel, L. L. & St Clair, R. W. Effects of LDL enriched with different dietary fatty acids on cholesteryl ester accumulation and turnover in THP-1 macrophages. J. Lipid Res. 44, 770–779 (2003).
Nakajima, K. et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1963–1972 (2011).
Wan, J.-B. et al. Endogenously decreasing tissue n-6/ n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 30, 2487–2494 (2010).
Leslie, M. A., Cohen, D. J. A., Liddle, D. M., Robinson, L. E. & Ma, D. W. L. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 14, 53 (2015).
Ito, M. K. Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: a review of the literature. Atherosclerosis 242, 647–656 (2015).
Franzese, C. J. et al. Relation of fish oil supplementation to markers of atherothrombotic risk in patients with cardiovascular disease not receiving lipid-lowering therapy. Am. J. Cardiol. 115, 1204–1211 (2015).
Yagi, S. et al. Effects of docosahexaenoic acid on the endothelial function in patients with coronary artery disease. J. Atheroscler. Thromb. 22, 447–454 (2015).
Tousoulis, D. et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 232, 10–16 (2014).
Burr, M. L. et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2, 757–761 (1989).
GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455 (1999).
Yokoyama, M., Origasa, H. & Matsuzaki, M. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 (2007); erratum 370, 220 (2007).
Niki, T. et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ. J. 80, 450–460 (2016).
Enns, J. et al. The impact of omega-3 polyunsaturated fatty acid supplementation on the incidence of cardiovascular events and complications in peripheral arterial disease: a systematic review and meta-analysis. BMC Cardiovasc. Disord. 14, 70 (2014).
Hooper, L. et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 332, 752–760 (2006).
Kwak, S. M., Myung, S. K., Lee, Y. J., Seo, H. G. & Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 172, 686–694 (2012).
Rizos, E. C., Ntzani, E. E., Bika, E., Kostapanos, M. S. & Elisaf, M. S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308, 1024–1033 (2012).
Iso, H. et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 113, 195–202 (2006).
Kobayashi, M., Sasaki, S., Kawabata, T., Hasegawa, K. & Tsugane, S. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess fatty acid intake: comparison with dietary records and serum phospholipid level. J. Epidemiol. 13, S64–S81 (2003).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01492361 (2016).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02104817 (2016).
Harris, W. S. et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119, 902–907 (2009).
Kakutani, S., Kawashima, H., Tanaka, T., Shiraishi-Tateishi, A. & Kiso, Y. Uptake of dihomo-γ-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot. Essent. Fatty Acids 83, 23–29 (2010).
Bai, W., Zheng, X., Zhou, L. & Li, H. Prostaglandin E1 dose-dependently promotes stability of atherosclerotic plaque in a rabbit model. Can. J. Physiol. Pharmacol. 90, 131–139 (2012).
Juan, H. & Sametz, W. Dihomo-δ-linolenic acid increases the metabolism of eicosapentaenoic acid in perfused vascular tissue. Prostaglandins Leukot. Med. 19, 79–86 (1985).
Takai, S. et al. Anti-atherosclerotic effects of dihomo-γ-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 16, 480–489 No430 (2009).
Engler, M. M. Comparative study of diets enriched with evening primrose, black currant, borage or fungal oils on blood pressure and pressor responses in spontaneously hypertensive rats. Prostaglandins Leukot. Essent. Fatty Acids 49, 809–814 (1993).
Luostarinene, R., Boberg, M. & Saldeen, T. Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis 99, 187–193 (1993).
Felton, C. V., Crook, D., Davies, M. J. & Oliver, M. F. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler. Thromb. Vasc. Biol. 17, 1337–1345 (1997).
Gautam, M. et al. Importance of fatty acid compositions in patients with peripheral arterial disease. PLoS ONE 9, e107003 (2014).
Leng, G. C. et al. Randomized controlled trial of gamma-linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clin. Nutr. 17, 265–271 (1998).
Guivernau, M., Meza, N., Barja, P. & Roman, O. Clinical and experimental study on the long-term effect of dietary gamma-linolenic acid on plasma lipids, platelet aggregation, thromboxane formation, and prostacyclin production. Prostaglandins Leukot. Essent. Fatty Acids 51, 311–316 (1994).
Tomiyama, H. et al. Relationships among the serum omega fatty acid levels, serum C-reactive protein levels and arterial stiffness/wave reflection in Japanese men. Atherosclerosis 217, 433–436 (2011).
Reinders, I. et al. Higher plasma phospholipid n-3 PUFAs, but lower n-6 PUFAs, are associated with lower pulse wave velocity among older adults. J. Nutr. 145, 2317–2324 (2015).
Szczeklik, A., Gryglewski, R. J., Sladek, K., Kostaka-Trabka, E. & Zmuda, A. Dihomo-γ-linolenic acid in patients with atherosclerosis: effects on platelet aggregation, plasma lipids and low-density lipoprotein-induced inhibition of prostacyclin generation. Thromb. Haemost. 51, 186–188 (1984).
Sluijs, I., Plantinga, Y., de Roos, B., Mennen, L. I. & Bots, M. L. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am. J. Clin. Nutr. 91, 175–183 (2010).
Ramsden, C. E. et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 353, i1246 (2016).
Smit, L. A., Baylin, A. & Campos, H. Conjugated linoleic acid in adipose tissue and risk of myocardial infarction. Am. J. Clin. Nutr. 92, 34–40 (2010).
Kromhout, D. et al. Food consumption patterns in the 1960s in seven countries. Am. J. Clin. Nutr. 49, 889–894 (1989).
Keys, A. Coronary heart disease in seven countries. Nutrition 13, 250–252; discussion 249, 253 (1997).
Scoditti, E. et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 527, 81–89 (2012).
Carluccio, M. A. et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 23, 622–629 (2003).
Soler-Rivas, C., Espín, J. C. & Wichers, H. J. Oleuropein and related compounds. J. Sci. Food Agric. 80, 1013–1023 (2000).
Dell'Agli, M. et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 54, 3259–3264 (2006).
Rosignoli, P., Fuccelli, R., Fabiani, R., Servili, M. & Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 24, 1513–1519 (2013).
Mangas-Cruz, M. A. et al. Effects of minor constituents (non-glyceride compounds) of virgin olive oil on plasma lipid concentrations in male Wistar rats. Clin. Nutr. 20, 211–215 (2001).
Gorinstein, S. et al. Olive oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. J. Agric. Food Chem. 50, 6102–6108 (2002).
González-Santiago, M. et al. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 188, 35–42 (2006).
Acin, S. et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. J. Biochem. 140, 383–391 (2006).
Covas, M. et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann. Intern. Med. 145, 333–341 (2006).
Fito, M. et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis 181, 149–158 (2005).
Gimeno, E. et al. Changes in the phenolic content of low density lipoprotein after olive oil consumption in men. A randomized crossover controlled trial. Br. J. Nutr. 98, 1243–1250 (2007).
Fito, M. et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur. J. Clin. Nutr. 62, 570–574 (2008).
Valls, R.-M. et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 167, 30–35 (2015).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368, 1279–1290 (2013).
Murie-Fernandez, M. et al. Carotid intima-media thickness changes with Mediterranean diet: a randomized trial (PREDIMED-Navarra). Atherosclerosis 219, 158–162 (2011).
Konstantinidou, V. et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: a randomized controlled trial. FASEB J. 24, 2546–2557 (2010).
Widmer, R. J. et al. Beneficial effects of polyphenol-rich olive oil in patients with early atherosclerosis. Eur. J. Nutr. 52, 1223–1231 (2013).
Wang, R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896 (2012).
Lee, D. Y. et al. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food 15, 992–999 (2012).
Zanardo, R. C. O. et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 20, 2118–2120 (2006).
Rinaldi, L. et al. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Invest. 86, 391–397 (2006).
Muzaffar, S. et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J. Vasc. Res. 45, 521–528 (2008).
Zhao, Z. Z. et al. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp. Biol. Med. (Maywood) 236, 169–176 (2011).
Zhang, H. et al. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur. J. Pharmacol. 697, 106–116 (2012).
Gonen, A. et al. The antiatherogenic effect of allicin: possible mode of action. Pathobiology 72, 325–334 (2005).
Koscienly, J. et al. The antiatherosclerotic effect of Allium sativum. Atherosclerosis 144, 237–249 (1999).
Budoff, M. et al. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: a preliminary study. Prev. Med. 39, 985–991 (2004).
Ackermann, R. T. et al. Garlic shows promise for improving some cardiovascular risk factors. Arch. Intern. Med. 161, 813 (2001).
Gardner, C. et al. Effect of raw garlic versus commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch. Intern. Med. 167, 346–353 (2007).
Katan, M. B. et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 78, 965–978 (2003).
Andersson, S. W. et al. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur. J. Clin. Nutr. 58, 1378–1385 (2004).
Sabeva, N. S. et al. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutr. Biochem. 22, 777–783 (2011).
Nashed, B., Yeganeh, B., HayGlass, K. & Moghadasian, M. Anti-atherogenic effects of dietary plant sterols are associated with inhibition of proinflammatory cytokine production in Apo E-KO mice. J. Nutr. 135, 2438–2444 (2005).
Xu, Z., Le, K. & Moghadasian, M. H. Long-term phytosterol treatment alters gene expression in the liver of apo E-deficient mice. J. Nutr. Biochem. 19, 545–554 (2008).
Moghadasian, M. H., McManus, B. M., Godin, D. V., Rodrigues, B. & Frohlich, J. J. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation 99, 1733–1739 (1999).
Yeganeh, B., Moshtaghi-Kashanian, G. R., Declercq, V. & Moghadasian, M. H. Combination of dietary phytosterols plus niacin or fenofibrate: effects on lipid profile and atherosclerosis in apo E-KO mice. J. Nutr. Biochem. 16, 222–228 (2005).
Moghadasian, M. H. Dietary phytosterols reduce cyclosporine-induced hypercholesterolemia in apolipoprotein E-knockout mice. Transplantation 81, 207–213 (2006).
Ras, R. T. et al. The effect of a low-fat spread with added plant sterols on vascular function markers: results of the Investigating Vascular Function Effects of Plant Sterols (INVEST) study. Am. J. Clin. Nutr. 101, 733–741 (2015).
Rocha, V. Z. et al. Effects of phytosterols on markers of inflammation: a systematic review and meta-analysis. Atherosclerosis 248, 76–83 (2016).
Gylling, H. et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 232, 346–360 (2014).
Stock, J. Focus on lifestyle: EAS Consensus Panel position statement on phytosterol-added foods. Atherosclerosis 234, 142–145 (2014).
Ottestad, I. et al. Phytosterol capsules and serum cholesterol in hypercholesterolemia: a randomized controlled trial. Atherosclerosis 228, 421–425 (2013).
Amir Shaghaghi, M., Abumweis, S. S. & Jones, P. J. H. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J. Acad. Nutr. Diet. 113, 1494–1503 (2013).
Chan, Y. et al. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr. Rev. 64, 385–402 (2006).
Rajaratnam, R. A., Gylling, H. & Miettinen, T. A. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35, 1185–1191 (2000).
Assmann, G. et al. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 16, 13–21 (2006).
Falcone Ferreyra, M. L., Rius, S. P. & Casati, P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3, 222 (2012).
Yamakuchi, M., Bao, C., Ferlito, M. & Lowenstein, C. J. Epigallocatechin gallate inhibits endothelial exocytosis. Biol. Chem. 389, 935–941 (2008).
Morrison, M. et al. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis 233, 149–156 (2014).
Fisher, N., Hughes, M., Gerhard-Herman, M. & Hollenberg, N. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 21, 2281–2286 (2003).
Velayutham, P., Babu, A. & Liu, D. M. Green tea catechins and cardiovascular health: an update. Curr. Med. Chem. 15, 1840–1850 (2008).
Tinahones, F. et al. Green tea reduces LDL oxidability and improves vascular function. J. Am. Coll. Nutr. 27, 209–213 (2008).
Matsuyama, T., Tanaka, Y., Kamimaki, I., Nagao, T. & Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity 101, 1338–1348 (2008).
Rassaf, T. et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial. Clin. J. Am. Soc. Nephrol. 11, 108–118 (2016).
Flammer, A. J. et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 33, 2172–2180 (2012).
Sansone, R. et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study. Br. J. Nutr. 114, 1246–1255 (2015).
Heiss, C. et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age 37, 9794 (2015).
Hsu, S. et al. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 86, 539–547 (2007).
Frank, J. et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J. Nutr. 139, 58–62 (2009).
West, S. G. et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 111, 653–661 (2014).
Osganian, S. K. et al. Vitamin C and risk of coronary heart disease in women. J. Am. Coll. Cardiol. 42, 246–252 (2003).
d'Uscio, L. V. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ. Res. 92, 88–95 (2002).
Matsumoto, T. et al. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. J. Pharm. Exp. Ther. 306, 103–108 (2003).
Averill, M. M. et al. Neither antioxidants nor genistein inhibit the progression of established atherosclerotic lesions in older apoE deficient mice. Atherosclerosis 203, 82–88 (2009).
Jiang, F., Jones, G. T. & Dusting, G. J. Failure of antioxidants to protect against angiotensin II-induced aortic rupture in aged apolipoprotein(E)-deficient mice. Br. J. Pharmacol. 152, 880–890 (2007).
Gavrila, D. et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Atheroscler. Thromb. Vasc. Biol. 25, 1671–1677 (2005).
Khaw, K. et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European prospective investigation into cancer and nutrition. Lancet 357, 657–663 (2001).
Ghanim, H. et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 91, 940–949 (2010).
Levine, G. N. et al. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93, 1107–1113 (1996).
Heitzer, T., Just, H. & Munzel, T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94, 6–9 (1996).
Ashor, A. W., Lara, J., Mathers, J. C. & Siervo, M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 235, 9–20 (2014).
Salonen, J. T. et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J. Intern. Med. 248, 377–386 (2000).
Knekt, P. et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am. J. Clin. Nutr. 80, 1508–1520 (2004).
Stephens, N. G. et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 347, 781–786 (1996).
Plantinga, Y. et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am. J. Hypertens. 20, 392–397 (2007).
Raitakari, O. T. et al. Oral vitamin C and endothelial function in smokers: short-term improvement, but no sustained beneficial effect. J. Am. Coll. Cardiol. 35, 1616–1621 (2000).
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 23–33 (2002).
Yusuf, S., Dagenais, G., Pogue, J., Bosch, J. & Sleight, P. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 342, 154–160 (2000).
Hodis, H. N. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 106, 1453–1459 (2002).
Kris-Etherton, P. M., Lichtenstein, A. H., Howard, B. V., Steinberg, D. & Witztum, J. L. Antioxidant vitamin supplements and cardiovascular disease. Circulation 110, 637–641 (2004).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Liu, T. et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 35, 1676–1684 (2012).
Miller, S. J., Zaloga, G. P., Hoggatt, A. M., Labarrere, C. & Faulk, W. P. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition 21, 740–748 (2005).
Menzel, T. et al. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 10, 122–128 (2004).
Aguilar, E. C. et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 24, 606–613 (2014).
Liu, S. et al. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J. Am. Coll. Cardiol. 39, 49–56 (2002).
Merchant, A. et al. Dietary fiber reduces peripheral arterial disease risk in men. J. Nutr. 133, 3658–3663 (2003).
Oh, K. et al. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. Am. J. Epidemiol. 161, 161–169 (2005).
Pereira, M. et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch. Intern. Med. 164, 370–376 (2004).
Ramos, S. C. et al. The role of soluble fiber intake in patients under highly effective lipid-lowering therapy. Nutr. J. 10, 1–8 (2011).
Kohen, R., Yamamoto, Y., Cundy, K. C. & Ames, B. N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl Acad. Sci. USA 85, 3175–3179 (1988).
Rashid, I., van Reyk, D. & Davies, M. Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro. FEBS Lett. 581, 1067–1070 (2007).
Brown, B. et al. Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E−/− mice. Atherosclerosis 232, 403–409 (2014).
Kim, M. Y., Kim, E. J., Kim, Y.-N., Choi, C. & Lee, B.-H. Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr. Res. Pract. 5, 421–428 (2011).
de Courten, B. et al. Effects of carnosine supplementation on glucose metabolism: pilot clinical trial. Obesity 24, 1027–1034 (2016).
Ghirlanda, G. et al. Evidence of plasma coq10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J. Clin. Pharmacol. 33, 226–229 (1993).
Wang, D. et al. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler. Thromb. Vasc. Biol. 34, 1860–1870 (2014).
Gairola, C. G., Howatt, D. A. & Daugherty, A. Dietary coenzyme Q10 does not protect against cigarette smoke-augmented atherosclerosis in apoE-deficient mice. Free Radic. Biol. Med. 48, 1535–1539 (2010).
Yan, X. et al. Coenzyme Q10 consumption promotes ABCG1-mediated macrophage cholesterol efflux: a randomized, double-blind, placebo-controlled, cross-over study in healthy volunteers. Mol. Nutr. Food Res. 59, 1725–1734 (2015).
Sanoobar, M. et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 18, 169–176 (2015).
Gao, L. et al. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis 221, 311–316 (2012).
Dai, Y.-L. et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis 216, 395–401 (2011).
Lee, Y. J., Cho, W. J., Kim, J. K. & Lee, D. C. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double-blind randomized controlled study. J. Med. Food 14, 386–390 (2011).
Zeb, I. et al. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: a randomized clinical trial. J. Cardiovasc. Dis. Res. 3, 185–190 (2012).
Larijani, V. N. et al. Beneficial effects of aged garlic extract and coenzyme Q10 on vascular elasticity and endothelial function: the FAITH randomized clinical trial. Nutrition 29, 71–75 (2013).
Abe, Y., Hashimoto, S. & Horie, T. Curcumin inhibitor of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 39, 41–47 (1999).
Gao, S. et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 85, 131–139 (2015).
Ramirez-Torosa, M. et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 147, 371–378 (1999).
Quiles, J. L. et al. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler. Thromb. Vasc. Biol. 22, 1225–1231 (2002).
Olszanecki, R. et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 56, 627–635 (2005).
Chuengsamarn, S., Rattanamongkolgul, S., Phonrat, B., Tungtrongchitr, R. & Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J. Nutr. Biochem. 25, 144–150 (2014).
Akazawa, N. et al. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 32, 795–799 (2012).
Anand, P., Kunnumakkara, A. B., Newman, R. A. & Aggarwal, B. B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807–818 (2007).
Arab, L. & Steck, S. Lycopene and cardiovascular disease. Am J. Clin. Nutr. 71, 1691S–1695S; discussion 1696S–1697S (2000).
Rao, A. V. & Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 19, 563–569 (2000).
Fuhrman, B., Elis, A. & Aviram, M. Hypercholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophage. Biochem. Biophys. Res. Commun. 233, 658–662 (1997).
Dugas, T. R., Morel, D. W. & Harrison, E. H. Impact of LDL carotenoid and α-tocopherol content on LDL oxidation by endothelial cells in culture. J. Lipid Res. 39, 999–1007 (1998).
Zou, Z.-Y. et al. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 111, 474–480 (2014).
Kim, O. Y. et al. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 208, 581–586 (2010).
Gajendragadkar, P. R. et al. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS ONE 9, e99070 (2014).
Thies, F. et al. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: a randomized controlled trial. Am. J. Clin. Nutr. 95, 1013–1022 (2012).
Richard, J. L. Coronary risk factors. The French paradox. Arch. Mal. Coeur Vaiss. 80, 17–21 (in French) (1987).
Voloshyna, I., Hai, O., Littlefield, M., Carsons, S. & Reiss, A. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur. J. Pharmacol. 698, 299–309 (2013).
Berbée, J. F. P. et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J. Nutr. Biochem. 24, 1423–1430 (2013).
Levantesi, G. et al. Wine consumption and risk of cardiovascular events after myocardial infarction: results from the GISSI-Prevenzione trial. Int. J. Cardiol. 163, 282–287 (2013).
Pirillo, A. & Catapano, A. L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis 243, 449–461 (2015).
Jeong, H. W. et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 296, E955–E964 (2009).
Cheng, W.-E. et al. Berberine reduces Toll-like receptor-mediated macrophage migration by suppression of Src enhancement. Eur. J. Pharmacol. 757, 1–10 (2015).
Lee, T.-S. et al. Anti-atherogenic effect of berberine on LXRα-ABCA1-dependent cholesterol efflux in macrophages. J. Cell. Biochem. 111, 104–110 (2010).
Wang, Q. et al. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS ONE 6, e25436 (2011).
Hu, Y. & Davies, G. E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 81, 358–366 (2010).
Li, H. et al. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284, 28885–28895 (2009).
Kong, W. et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10, 1344–1351 (2004).
Derosa, G. et al. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin. Biol. Ther. 13, 475–482 (2013).
Kong, W.-J. et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 57, 1029–1037 (2008).
Affuso, F., Ruvolo, A., Micillo, F., Saccà, L. & Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 20, 656–661 (2010).
Acknowledgements
Our research was supported by grants from the British Heart Foundation (PG/07/031/22716, PG/08/073/25520, PG/10/55/28467 and PG/12/50/29691). We apologize to all the authors whose work could not be cited because of space limitations, and thank Daryn R. Michael (Cultech Limited, UK) for his valuable input.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Moss, J., Ramji, D. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol 13, 513–532 (2016). https://doi.org/10.1038/nrcardio.2016.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.103
This article is cited by
-
Additive interaction between potentially modifiable risk factors and ethnicity among individuals in the Han, Tujia and Miao populations with first-ever ischaemic stroke
BMC Public Health (2021)
-
Phytoconstituents of an ethanolic pod extract of Prosopis cineraria triggers the inhibition of HMG-CoA reductase and the regression of atherosclerotic plaque in hypercholesterolemic rabbits
Lipids in Health and Disease (2020)
-
MiR-128-3p inhibits vascular smooth muscle cell proliferation and migration by repressing FOXO4/MMP9 signaling pathway
Molecular Medicine (2020)
-
Kefir peptides alleviate high-fat diet-induced atherosclerosis by attenuating macrophage accumulation and oxidative stress in ApoE knockout mice
Scientific Reports (2020)
-
Cholesterol-Lowering Effects of Lactobacillus Species
Current Microbiology (2020)