Key Points
-
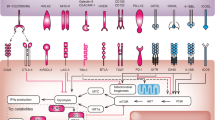
Exploration for biomarkers for drugs that block immune checkpoints should be rationally conducted based on knowledge of the mechanism of action of the targeted pathway. The programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte associated antigen 4 (CTLA4) pathways are unique, and there are special considerations based on mechanisms of action for developing biomarkers for drugs blocking each of these pathways.
-
Biomarkers for immune checkpoint-blocking drugs currently fall into three major categories: immunological, genetic and virological. Future work may reveal additional markers related to metabolism and the microbiome.
-
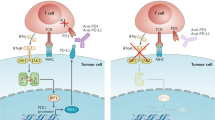
Immunological biomarkers offer the advantage of applicability across multiple tumour types amenable to immune checkpoint blockade. In the case of anti-PD1 drugs, tumour PD1 ligand 1 (PDL1) expression is a pretreatment biomarker that predicts a greater likelihood of response to therapy. Despite technical pitfalls that make clinical application challenging, two PDL1 immunohistochemistry tests are currently approved by the US Food and Drug Administration for guiding treatment decisions in patients with non-small-cell lung cancer and melanoma.
-
Although no specific oncogene or driver mutation has yet been correlated with clinical response to immune checkpoint blockade, overall tumour mutational burden reflecting neoantigenic diversity may have predictive value. This is exemplified by the high anti-PD1 response rate in DNA mismatch repair deficient colorectal cancers (which have a large mutational burden and which account for ∼15% of all colon cancers), whereas mismatch repair proficient colon cancers are unlikely to respond.
-
Virus-associated cancers, which account for more than 20% of cancers worldwide, express viral neoantigens that are strongly immunogenic. Early evidence demonstrates expression of PD1–PDL1 in these cancers, and suggests responsiveness to anti-PD1 therapies.
-
Combination treatment regimens based on immune checkpoint-blocking drugs are emerging as the next step in clinical development to improve efficacy and response durability. Biomarker considerations for these regimens are complex and are likely to involve multifactorial assessments.
Abstract
With recent approvals for multiple therapeutic antibodies that block cytotoxic T lymphocyte associated antigen 4 (CTLA4) and programmed cell death protein 1 (PD1) in melanoma, non-small-cell lung cancer and kidney cancer, and additional immune checkpoints being targeted clinically, many questions still remain regarding the optimal use of drugs that block these checkpoint pathways. Defining biomarkers that predict therapeutic effects and adverse events is a crucial mandate, highlighted by recent approvals for two PDL1 diagnostic tests. Here, we discuss biomarkers for anti-PD1 therapy based on immunological, genetic and virological criteria. The unique biology of the CTLA4 immune checkpoint, compared with PD1, requires a different approach to biomarker development. Mechanism-based insights from such studies may guide the design of synergistic treatment combinations based on immune checkpoint blockade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Pardoll, D. Cancer and the immune system: basic concepts and targets for intervention. Semin. Oncol. 42, 523–538 (2015).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Garon, E. et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Brahmer, J. R. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Motzer, R. J. et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 33, 1430–1437 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Lipson, E. J. et al. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin. Oncol. 42, 587–600 (2015).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012). The report of this large early-phase clinical study demonstrates a role for anti-PD1 therapy in NSCLC, melanoma and kidney cancer, but not in prostate cancer or CRC; it also provides the first demonstration of PDL1 IHC as a potential biomarker for anti-PD1 therapy.
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Schadendorf, D. et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894 (2015).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Brahmer, J. R. et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010). This first-in-human study of MDX-1106 (nivolumab) reports early evidence for clinical activity in multiple cancer types, pharmacodynamics and PDL1 IHC as a predictor of clinical outcomes.
Robert, L. et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 3, e29244 (2014).
Maker, A. V., Attia, P. & Rosenberg, S. A. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 175, 7746–7754 (2005).
Ku, G. Y. et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116, 1767–1775 (2010).
Yang, A. et al. CTLA-4 blockade with ipilimumab increases peripheral CD8+ T cells: correlation with clinical outcomes. J. Clin. Oncol. 28 (15 suppl.), 2555 (2010).
Yuan, J. et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl Acad. Sci. USA 105, 20410–20415 (2008).
Yuan, J. et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc. Natl Acad. Sci. USA 108, 16723–16728 (2011).
Hannani, D. et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 25, 208–224 (2015).
Ji, R. R. et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol. Immunother. 61, 1019–1031 (2012).
Chen, H. et al. Anti-CTLA-4 therapy results in higher CD4+ ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc. Natl Acad. Sci. USA 106, 2729–2734 (2009).
Vonderheide, R. H. et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 16, 3485–3494 (2010).
Hodi, F. S. et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA 105, 3005–3010 (2008).
Liakou, C. I. et al. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105, 14987–14992 (2008).
Virchow, R. Die Krankhaften Geschwülste (Berlin, 1863).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Fridman, W. H., Pagès, F., Sautès-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Dieu-Nosjean, M. C. et al. Long term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Di Caro, G. et al. Occurrence of tertiary lymphoid tissue is associated to T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 20, 2147–2158 (2014).
Taube, J. M. Emerging immunologic biomarkers: setting the (TNM-immune) stage. Clin. Cancer Res. 20, 2023–2025 (2014).
Dong, H., Zhu, G., Tamada, K. & Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369 (1999).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Mazanet, M. M. & Hughes, C. C. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169, 3581–3588 (2002).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Green, M. R. et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116, 3268–3277 (2010).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Taube, J. M. et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl Med. 4, 127ra37 (2012). This report provides the first evidence for, and articulation of, the adaptive immune resistance hypothesis.
Taube, J. M. et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin. Cancer Res. 21, 3969–3976 (2015).
Lipson, E. J. et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol. Res. 1, 54–63 (2013).
Velcheti, V. et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest. 94, 107–116 (2014).
Schalper, K. A. et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin. Cancer Res. 20, 2773–2782 (2014).
Cimino-Mathews, A. et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 47, 52–63 (2016).
Lyford-Pike, S. et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 73, 1733–1741 (2013).
Skoulidis, F. et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 5, 860–877 (2015).
Rodic, N. et al. PD-L1 expression in melanocytic lesions does not correlate with the BRAF V600E mutation. Cancer Immunol. Res. 3, 110–115 (2015).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Llosa, N. J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66, 3381–3385 (2006).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Lipson, E. J. et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 19, 462–468 (2013).
Thompson, E. D. et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut http://dx.doi.org/10.1136/gutjnl-2015-310839, (2016).
Sunshine, J. & Taube, J. M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 23, 32–38 (2015).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
McDermott, D. F. et al. Atezolizumab, and anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity and immune correlates from a phase Ia study. J. Clin. Oncol. 34, 833–842 (2016).
Kitazono, S. et al. Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in non-small-cell lung cancer. Clin. Lung Cancer 16, 385–390 (2015).
Dabbs, D. J. in Diagnostic immunohistochemistry (ed. Dabbs, D. J.) 29–39 (Churchill Livingstone, 2002).
Von Mehren, M. et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin. Cancer Res. 7, 1181–1189 (2001).
Emens, L. A. & Middleton, G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res. 3, 436–443 (2015).
Garraway, L. A. Genomics-driven oncology: framework for an emerging paradigm. J. Clin. Oncol. 31, 1806–1814 (2013).
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006).
Marzec, M. et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl Acad. Sci. USA 105, 20852–20857 (2008).
Akbay, E. A. et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3, 1355–1363 (2013).
Xu, C. et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25, 590–604 (2014).
Larkin, J. et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 1, 433–440 (2015).
Weber, J. S. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
Hellmann, M. D. et al. Efficacy of pembrolizumab in key subgroups of patients with advanced NSCLC. J. Thor. Oncol. 10 (suppl. 2) abstract MINI03.05 (2015).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science. 348, 69–74 (2015).
Shastri, N., Cardinaud, S., Schwab, S. T., Serwold, T. & Kunisawa, J. All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol. Rev. 207, 31–41 (2005).
Roche, P. A. & Furata, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 (2015).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012). This article reports the first demonstration of clinical activity of a drug to block PDL1; a companion article in the same journal issue (ref. 14) describes a different drug that blocks PD1; together these papers highlight the PD1–PDL1 pathway as a vital target in cancer immunotherapy.
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Van Allen, E. M. et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014).
Tran, E. et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390 (2015).
Kvistborg, P. et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl Med. 6, 254ra128 (2014).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Coulie, P. G., Van den Eynde, B. J., van der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Liu, B. et al. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat. Med. 2, 169–174 (1996).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Drescher, K. M. et al. Lymphocyte recruitment into the tumor site is altered in patients with MSI-H colon cancer. Fam. Cancer 8, 231–239 (2009).
Le, D. T. et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015). This is the first report linking a genetic marker in cancer (MSI) with clinical outcomes following anti-PD1 therapy.
Sukowati, C. H. et al. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J. Gastroenterol. 22, 1497–1512 (2016).
Pearson, G. R. Epstein-Barr virus and nasopharyngeal carcinoma. J. Cell. Biochem. Suppl. 17, 150–154 (1993).
Stanley, M. A. Human papillomavirus vaccines. Rev. Med. Virol. 16, 139–149 (2006).
Hoots, B. E., Palefsky, J. M., Pimenta, J. M. & Smith, J. S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Cancer 124, 2375–2383 (2009).
Matsuoka, M. Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology 2, 27 (2005).
Wen, K. W. & Damania, B. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett. 289, 140–150 (2010).
Chen, X. Z., Chen, H., Castro, F. A., Hu, J. K. & Brenner, H. Epstein–Barr virus infection and gastric cancer: a systematic review. Medicine 94, e792 (2015).
List, A. F., Greco, F. A. & Vogler, L. B. Lymphoproliferative diseases in immunocompromised hosts: the role of Epstein–Barr virus. J. Clin. Oncol. 5, 1673–1689 (1987).
Kapatai, G. & Murray, P. Contribution of the Epstein–Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J. Clin. Pathol. 60, 1342–1349 (2007).
Samimi, M. et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin. Oncol. 42, 347–358 (2015).
Gillison, M. L. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst. 92, 709–720 (2000).
Nghiem, P. et al. Activity of PD-1 blockade with pembrolizumab as first systemic therapy in patients with advanced Merkel cell carcinoma. Ann. Oncol. 27 (suppl.), abstract 22LBA, in the press (2015).
El-Khoueiry, A. B. et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J. Clin. Oncol. 33 (15 suppl.), abstract LBA101 (2015).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Melero, I. et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). This initial report demonstrates the activity and side effect profile of anti-PD1 plus anti-CTLA4 therapy, the first immunotherapy combination to eventually receive FDA approval.
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015).
Goldberg, M. V. & Drake, C. G. LAG-3 in cancer immunotherapy. Curr. Top. Microbiol. Immunol. 344, 269–278 (2011).
Ngiow, S. F. et al. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71, 3540–3551 (2011).
Yi, K. H. & Chen, L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol. Rev. 229, 145–151 (2009).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Zarek, P. E. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259 (2008).
Uyttenhove, C. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9, 1269–1274 (2003).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Nishimura, H. et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Acknowledgements
The authors are grateful to M. Hellmann (Memorial Sloan-Kettering Cancer Center, New York, USA), E. Garon (University of California Los Angeles, USA) and J. Brahmer (Johns Hopkins University, Baltimore, Maryland, USA) for helpful discussions. This work was supported by research funding from Bristol-Myers Squibb (S.L.T., J.M.T., R.A.A. and D.M.P.), the Melanoma Research Alliance (S.L.T., J.M.T. and D.M.P.), the US National Cancer Institute NIH (R01 CA142779; S.L.T., J.M.T. and D.M.P.), the Barney Family Foundation (S.L.T. and J.M.T.), the Dermatology Foundation (J.M.T.), the Laverna Hahn Charitable Trust (S.L.T.), the Commonwealth Foundation (D.M.P.), the W.W. Smith Charitable Trust (J.M.T.) and Moving for Melanoma Delaware (S.L.T., J.M.T. and D.M.P.). All authors were also supported by a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a programme of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.L.T., research grants from Bristol-Myers Squibb, and consulting for Five Prime Therapeutics, GlaxoSmithKline and Jounce Therapeutics. J.M.T., research support from Bristol-Myers Squibb, and consulting for Bristol-Myers Squibb, Merck and AstraZeneca. R.A.A., research support from Five Prime Therapeutics and Bristol-Myers Squibb, and consulting for Adaptive Biotechnologies. D.M.P., research grants from Bristol-Myers Squibb and Potenza Therapeutics; consulting for Amgen, Five Prime Therapeutics, GlaxoSmithKline, Jounce Therapeutics, MedImmune, Merck, Pfizer, Potenza Therapeutics, Sanofi and Tizona; stock options in Jounce, Potenza and Tizona; and patent royalties through his institution, from AstraZeneca, Bristol-Myers Squibb and Potenza.
Related links
Glossary
- Tolerance
-
An immunological phenomenon in which antigen-specific T and/or B cells are absent or unresponsive to antigen-bearing cells, as opposed to rejection, in which antigen-specific immune cells eliminate their targets.
- Mixed tumour regression
-
A therapeutic response pattern in which different metastatic lesions in an individual patient show different responses to therapy, some regressing whereas others progress.
- Regulatory T cells
-
(Treg cells). A subset of CD4+ T cells characterized by expression of the forkhead box transcription factor FOXP3, which interacts with other immune cells to inhibit immune responses.
- Adaptive immune system
-
Comprises T and B lymphocytes with unique antigen receptors generated by somatic DNA recombination events, as compared with the innate immune system, which comprises cells with invariant antigen receptors (for example, natural killer cells and macrophages).
- Tertiary lymphoid structure
-
Ectopic lymphoid tissue that recapitulates some of the structural organization of a lymph node (including T cells, B cells, antigen presenting cells and high endothelial venules) and can support the generation of an adaptive immune response.
- Adaptive immune resistance
-
A phenomenon in which tumour and stromal cells adapt to attack from infiltrating T cells by expressing the immune inhibitory ligand programmed cell death 1 ligand 1 (PDL1). In this scenario, PDL1 expression is driven by inflammatory cytokines such as interferon-γ secreted by tumour antigen-specific T cells.
- Objective response rate
-
The rate of significant tumour regressions in patients undergoing cancer therapy, including complete and partial regressions as defined by standard oncological criteria (for example, Response Evaluation Criteria In Solid Tumours (RECIST), or World Health Organization (WHO) criteria).
- Neoantigens
-
Newly expressed tumour antigens that arise from genetic alterations in tumour cells and are therefore not present in normal cells, such as antigens generated by somatic (non-heritable) mutations or oncoviruses.
- Self-antigen
-
In the context of tumour antigens, a non-mutated component of tumour cells that is also expressed by some normal cells and can be recognized by the immune system.
- Anergy
-
A functional state of T cells in which they are hyporesponsive to T cell receptor engagement by cognate antigen, relative to naive or memory T cells.
- DNA mismatch repair complex
-
(MMR complex). A complex of enzymes that recognizes DNA base mismatches introduced during DNA replication, excises them and replaces them with correctly matched bases.
- Microsatellite instability
-
(MSI). A hallmark of defects in DNA mismatch repair, characterized by alterations in the frequency of repeated DNA sequences (microsatellites).
- TH1 phenotype
-
Differentiation state of CD4+ T cells characterized by production of interferon-γ (in addition to other cytokines) upon encountering cognate antigen.
Rights and permissions
About this article
Cite this article
Topalian, S., Taube, J., Anders, R. et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16, 275–287 (2016). https://doi.org/10.1038/nrc.2016.36
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.36
This article is cited by
-
Identification and validation of an individualized metabolic prognostic signature for predicting the biochemical recurrence of prostate cancer based on the immune microenvironment
European Journal of Medical Research (2024)
-
Intratumoral microorganisms in tumors of the digestive system
Cell Communication and Signaling (2024)
-
ARID1A deficiency promotes progression and potentiates therapeutic antitumour immunity in hepatitis B virus-related hepatocellular carcinoma
BMC Gastroenterology (2024)
-
TRIP6 a potential diagnostic marker for colorectal cancer with glycolysis and immune infiltration association
Scientific Reports (2024)
-
Immune microenvironment heterogeneity of concurrent adenocarcinoma and squamous cell carcinoma in multiple primary lung cancers
npj Precision Oncology (2024)