Key Points
-
The so-called targeted therapies and cancer immunotherapies are two novel treatment modalities that have recently begun to enter the oncology clinic. Targeted therapies and immunotherapy offer a number of possible synergies in treatment when used together; however, these combinations have not been well studied.
-
Many targeted therapies against tumours affect pathways that are also crucial for immune development and function, which suggests the possibility that targeted therapies may help to optimize anti-tumour immune responses from immunotherapies. Similarly, immunotherapies may serve to consolidate impressive clinical responses from targeted therapies into long-lasting clinical remissions.
-
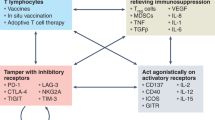
Targeted therapies promote effective dendritic cell (DC) maturation, T cell priming, activation and differentiation into long-lived memory T cells, which suggests possible combinations of cancer vaccines along with targeted therapies to bolster vaccine responses, as well as effector T cell function.
-
Targeted therapies may sensitize tumour cells to immune-mediated killing by increasing the expression of death receptors or 'distress' ligands while simultaneously diminishing the expression of pro-survival signals, which increases the efficiency of immune-mediated tumour clearance once immune cells are activated in vivo.
-
Targeted therapies might diminish tumour-mediated immunosuppression by abrogating the production of tumorigenic inflammation and by inhibiting immunosuppressive cell types. Impairing immunosuppression improves effector T cell function and increases immune destruction of tumour targets, suggesting possible synergy with immunotherapies that are designed to generate anti-tumour T cells or to bolster their effector function.
-
Important considerations regarding optimizing dose, sequence and timing of targeted therapies will be required when rationally designing future clinical trials in order to maximize anti-tumour efficacy while minimizing any immunosuppressive side effects.
Abstract
During the past two decades, the paradigm for cancer treatment has evolved from relatively nonspecific cytotoxic agents to selective, mechanism-based therapeutics. Cancer chemotherapies were initially identified through screens for compounds that killed rapidly dividing cells. These drugs remain the backbone of current treatment, but they are limited by a narrow therapeutic index, significant toxicities and frequently acquired resistance. More recently, an improved understanding of cancer pathogenesis has given rise to new treatment options, including targeted agents and cancer immunotherapy. Targeted approaches aim to inhibit molecular pathways that are crucial for tumour growth and maintenance; whereas, immunotherapy endeavours to stimulate a host immune response that effectuates long-lived tumour destruction. Targeted therapies and cytotoxic agents also modulate immune responses, which raises the possibility that these treatment strategies might be effectively combined with immunotherapy to improve clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Druker, B. J. David A. Karnofsky Award lecture. Imatinib as a paradigm of targeted therapies. J. Clin. Oncol. 21, 239s–245s (2003).
O'Brien, S. G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Haber, D. A., Gray, N. S. & Baselga, J. The evolving war on cancer. Cell 145, 19–24 (2011).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Korman, A., Peggs, K. & Allison, J. P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 293–335 (2006).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Rakhra, K. et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).
Chiarle, R. et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nature Med. 14, 676–680 (2008).
Farsaci, B., Higgins, J. P. & Hodge, J. W. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int. J. Cancer 8 Aug 2011 (doi:10.1002/ijc.26219). This paper details how alterations in the scheduling of the targeted therapy sunitinib significantly alter T Reg cell populations, and that pretreating with sunitinib improves vaccine efficacy in animal models; whereas, co-administration had no effect on vaccine efficacy.
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Nefedova, Y. et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J. Immunol. 175, 4338–4346 (2005).
Nefedova, Y. et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535 (2005). This paper discusses the use of a JAK2 inhibitor to improve the maturation of DCs, showing that animals treated with JAK2 inhibitors have increased numbers of mature DCs, increased T cell priming by DCs and have increased surival when the inhibitor was combined with a DC vaccine.
Seeger, J. M. et al. The proteasome inhibitor bortezomib sensitizes melanoma cells toward adoptive CTL attack. Cancer Res. 70, 1825–1834 (2010).
Hahnel, P. S. et al. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 68, 3899–3906 (2008).
Steinman, R. M. & Mellman, I. Immunotherapy: bewitched, bothered, and bewildered no more. Science 305, 197–200 (2004).
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
May, K. F. Jr, Chen, L., Zheng, P. & Liu, Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 62, 3459–3465 (2002).
Melero, I. et al. Monoclonal antibodies against the 4–1BB T-cell activation molecule eradicate established tumors. Nature Med. 3, 682–685 (1997).
Miller, R. E. et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J. Immunol. 169, 1792–1800 (2002).
Mitsui, J. et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 16, 2781–2791 (2010).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Li, B. et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor-secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin. Cancer Res. 15, 1623–1634 (2009).
Hodi, F. S. et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA 105, 3005–3010 (2008).
Boruchov, A. M. et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 115, 2914–2923 (2005).
Dougan, M. & Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 27, 83–117 (2009).
Correale, P. et al. Cetuximab ± chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int. J. Cancer 130, 1577–1589 (2012).
Wolpoe, M. E. et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J. Immunol. 171, 2161–2169 (2003).
Ladoire, S. et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br. J. Cancer 105, 366–371 (2011).
Park, S. et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 18, 160–170 (2010).
Kim, P. S. et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J. Clin. Invest. 118, 1700–1711 (2008).
Disis, M. L. et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 27, 4685–4692 (2009).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011). This paper demonstrates how targeted monoclonal antibody therapies, such as HER2 antibodies, require immune-mediated tumour destruction for clinical responses and synergize with both co-stimulatory 4-1BB agonistic antibodies, as well as blockade of an inhibitory signal through a PD1 antibody.
Jaime-Ramirez, A. C. et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production. J. Immunol. 186, 3401–3409 (2011).
Bekaii-Saab, T. S. et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol. Cancer Ther. 8, 2983–2991 (2009).
Marechal, R. et al. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer 10, 340 (2010).
Dechant, M. et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 68, 4998–5003 (2008).
Hsu, Y. F. et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol. Cancer 9, 139 (2010).
Lee, H., Pal, S. K., Reckamp, K., Figlin, R. A. & Yu, H. STAT3: a target to enhance antitumor immune response. Curr. Top. Microbiol. Immunol. 344, 41–59 (2011).
Kilinc, M. O., Gu, T., Harden, J. L., Virtuoso, L. P. & Egilmez, N. K. Central role of tumor-associated CD8+ T effector/memory cells in restoring systemic antitumor immunity. J. Immunol. 182, 4217–4225 (2009).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Leffers, N. et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 58, 449–459 (2009).
Araki, K., Ellebedy, A. H. & Ahmed, R. TOR in the immune system. Curr. Opin. Cell Biol. 23, 707–715 (2011).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009). This paper illustrates how inhibitors of the mTOR pathway, such as rapamycin, enhance memory T cell differentiation and augment their function in multiple different animal models of viral infection.
Wang, Y., Wang, X. Y., Subjeck, J. R., Shrikant, P. A. & Kim, H. L. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br. J. Cancer 104, 643–652 (2011).
Jiang, Q. et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 71, 4074–4084 (2011).
Procaccini, C. et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33, 929–941 (2010).
Wang, Y. et al. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J. Immunol. 186, 2809–2818 (2011).
Mai, W. et al. Deregulated GSK3β sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin. Cancer Res. 15, 6810–6819 (2009).
Shakoori, A. et al. Inhibition of GSK-3 β activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 98, 1388–1393 (2007).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nature Med. 17, 1290–1297 (2011). This paper demonstrates how targeted therapies, such as GSK3β inhibitors, are able to drive T cell differentiation to retain long-lasting, self-renewing, T scm cells that provide potent tumour protection in adoptive T cell transfer models.
Fesik, S. W. Promoting apoptosis as a strategy for cancer drug discovery. Nature Rev. Cancer 5, 876–885 (2005).
Dougan, M. et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J. Exp. Med. 207, 2195–2206 (2010). This paper shows that IAP inhibitors increase T cell responses to multiple different immune stimuli in vitro and that combining IAP inhibitors with tumour vaccination decreases tumour growth kinetics.
Varfolomeev, E. & Vucic, D. (Un)expected roles of c-IAPs in apoptotic and NFκB signaling pathways. Cell Cycle 7, 1511–1521 (2008).
Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Richardson, P. G., Mitsiades, C., Hideshima, T. & Anderson, K. C. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med. 57, 33–47 (2006).
Shi, J. et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood 111, 1309–1317 (2008).
Hallett, W. H. et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J. Immunol. 180, 163–170 (2008).
Chen, L. et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005).
Tseng, C. W. et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J. Mol. Med. 86, 899–908 (2008).
Noh, K. H. et al. Activation of Akt as a mechanism for tumor immune evasion. Mol. Ther. 17, 439–447 (2009). This paper demonstrates that one mechanism of tumour resistance to vaccination therapy is through the upregulation of the AKT pathway, which mediates resistance to apoptosis; inhibiting this pathway increased CTL-mediated killing of AKT-upregulated tumour cells in vitro and AKT inhibition in combination with vaccination augmented responses
Boni, A. et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70, 5213–5219 (2010). This paper shows how targeted inhibition of mutant BRAF augments expression of tumour antigens on the tumour cell surface, increasing T cell responses against tumour cells while showing no deleterious effect on T cell proliferation or function.
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Taipale, M., Jarosz, D. F. & Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature Rev. Mol. Cell Biol. 11, 515–528 (2010).
Lin, C. C. et al. Inhibitor of heat-shock protein 90 enhances the antitumor effect of DNA vaccine targeting clients of heat-shock protein. Mol. Ther. 15, 404–410 (2007).
Kawabe, M. et al. Heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin enhances EphA2+ tumor cell recognition by specific CD8+ T cells. Cancer Res. 69, 6995–7003 (2009).
Boll, B. et al. Heat shock protein 90 inhibitor BIIB021 (CNF2024) depletes NF-κB and sensitizes Hodgkin's lymphoma cells for natural killer cell-mediated cytotoxicity. Clin. Cancer Res. 15, 5108–5116 (2009).
Fionda, C. et al. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J. Immunol. 183, 4385–4394 (2009).
Gasser, S. & Raulet, D. H. The DNA damage response arouses the immune system. Cancer Res. 66, 3959–3962 (2006).
Poggi, A. et al. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia 23, 641–648 (2009).
Skov, S. et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 65, 11136–11145 (2005).
Rabinovich, G. A., Gabrilovich, D. & Sotomayor, E. M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Alfaro, C. et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br. J. Cancer 100, 1111–1119 (2009).
Yang, D. H. et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk. Res. 33, 665–670 (2009).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Ozao-Choy, J. et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 69, 2514–2522 (2009). This paper demonstrates how a targeted therapy, sunitinib, is able to decrease both the number and function of suppressive cells (T Reg cells and MDSCs) in tumour-infiltrating lymphocytes in an in vivo mouse model of colon cancer, and that combining sunitinib with agonistic 4-1BB antibodies and IL-12 improved responses to therapy.
Bose, A. et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int. J. Cancer 129, 2158–2170 (2011).
Hipp, M. M. et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111, 5610–5620 (2008).
Kujawski, M. et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 70, 9599–9610 (2010).
Avella, D. M. et al. Regression of established hepatocellular carcinoma is induced by chemo-immunotherapy in an orthotopic murine model. Hepatology 55, 141–152 (2011).
Green, M. R. et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116, 3268–3277 (2010).
Hwu, P. et al. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164, 3596–3599 (2000).
Balachandran, V. P. et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nature Med. 17, 1094–1100 (2011). This paper shows how imatinib blocks the supressor function of MDSCs through the inhibition of IDO, and that combining imatinib with CTLA4 antibodies improves clinical responses.
Larmonier, N. et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J. Immunol. 181, 6955–6963 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Schmid, M. C. et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell 19, 715–727 (2011). This paper demonstrates how pharmacological and genetic inhibition of the PI3K pathway impedes immune-mediated tumour-promoting inflammation, which slowed tumour growth.
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
Zitvogel, L., Kepp, O. & Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nature Rev. Clin. Oncol. 8, 151–160 (2011).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Kamrava, M., Bernstein, M. B., Camphausen, K. & Hodge, J. W. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol. Biosyst. 5, 1262–1270 (2009).
Bae, J. et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J. Immunol. 178, 7730–7737 (2007).
Yun, T. J. et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J. Immunol. 186, 563–575 (2011).
Feng, X. et al. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol. Immunol. 47, 2388–2396 (2010).
Wang, X. et al. Proteasome inhibition induces apoptosis in primary human natural killer cells and suppresses NKp46-mediated cytotoxicity. Haematologica 94, 470–478 (2009).
Rossi, L. E. et al. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J. Leukoc. Biol. 91, 321–331 (2011).
Dranoff, G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nature Rev. Immunol. 12, 61–66 (2011).
Kocak, E. et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 66, 7276–7284 (2006).
Curran, M. A., Montalvo, W., Yagita, H. & Allison, J. P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107, 4275–4280 (2010).
Topp, M. S. et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 (2011).
Burris, H. A. et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2011).
Zhang, B. et al. Immune surveillance and therapy of lymphomas driven by ebstein-barr-virus Protein LMP1 in a mouse model. Cell (in the press).
Halama, N. et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 71, 5670–5677 (2011).
Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010).
Ghiringhelli, F. et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34, 336–344 (2004).
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 64, 2205–2211 (2004).
Kimura, Y. et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas (2011).
Quoix, E. et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 12, 1125–1133 (2011).
Catellani, S., Pierri, I., Gobbi, M., Poggi, A. & Zocchi, M. R. Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia. PLoS ONE 6, e18925 (2011).
Preudhomme, C. et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N. Engl. J. Med. 363, 2511–2521 (2010).
Jain, N. et al. Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: a phase 2 trial. Cancer 115, 3924–3934 (2009).
Menard, C. et al. Natural killer cell IFN-γ levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 69, 3563–3569 (2009). This paper demonstrates that the presence of NK cells in patients with gastrointestinal stromal tumour treated with imatinib serves as an independent prognostic factor of clinical response, suggesting that off-target effects of imatinib that stimulate NK cells may partially account for its therapeutic success.
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Manzoni, M. et al. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology 79, 187–196 (2010).
Lee, S. C., Srivastava, R. M., Lopez-Albaitero, A., Ferrone, S. & Ferris, R. L. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol. Res. 50, 248–254 (2011).
Pander, J. et al. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin. Cancer Res. 17, 5668–5673 (2011).
Lanuti, P. et al. Enhancement of TRAIL cytotoxicity by AG-490 in human ALL cells is characterized by downregulation of cIAP-1 and cIAP-2 through inhibition of Jak2/Stat3. Cell Res. 19, 1079–1089 (2009).
Benson, D. M. Jr, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010).
Chanan-Khan, A. A. et al. Biological effects and clinical significance of lenalidomide-induced tumour flare reaction in patients with chronic lymphocytic leukaemia: in vivo evidence of immune activation and antitumour response. Br. J. Haematol. 155, 457–467 (2011).
Gorgun, G. et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 116, 3227–3237 (2010).
Herman, S. E. et al. The role of phosphatidylinositol 3-kinase-δ in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood 117, 4323–4327 (2011).
Benson, D. M. Jr, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 118, 6387–6391 (2011).
Wang, H. et al. IFN-β production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-β. J. Immunol. 181, 6797–6802 (2008).
Pardoll, D. M. Blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 12, 252–264 (2012).
Acknowledgements
Glenn Dranoff is supported by grants from the US National Cancer Institute, the Leukemia and Lymphoma Society, the Melanoma Research Alliance, the Alliance for Cancer Gene Therapy, the Research Foundation for the Treatment of Ovarian Cancer, and Novartis Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.D. is a consultant and receives sponsored research support from Novartis Inc.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Complete cytogenetic responses
-
The lack of any detectable tumour burden by conventional cytogenetic studies, such as karyotype analysis or fluorescence in situ hybridization (FISH).
- Dendritic cell (DC) vaccine
-
A process by which DCs are removed from the patient, loaded with tumour or tumour antigens, matured and then re-infused back into the patient to stimulate immune responses in vivo.
- Oncogene addiction
-
A process by which a single mutated gene or signalling pathway drives tumour proliferation; inhibition of that gene or pathway results in rapid tumour response.
- Vaccination in situ
-
As tumour cells die and release 'danger' molecules, tumour antigens are phagocytosed and presented by dendritic cells to prime anti-tumour immune responses.
- Regulatory T (TReg) cells
-
A T cell subtype that releases suppressive cytokines and serves to silence immune responses.
- Myeloid-derived suppressor cells
-
MDSCs. A myeloid cell subtype that silences responses of cytotoxic CD8+ T cells and helper CD4+ T cells while simultaneously promoting the formation of regulatory T cells.
- Major histocompatibility complex
-
MHC. Proteins that are responsible for displaying varied peptide antigens on the cell surface.
- CD8+ T cells
-
A T cell subtype that recognizes a specific peptide on target cells and kills those cells.
- CD4+ T cells
-
A T cell subtype that recognizes peptides on target cells and secretes signalling molecules (called cytokines) to direct an appropriate immune response.
- Anergy
-
A state in which T cells do not respond to antigenic stimulation even when presented in the appropriate context.
- Exhaustion
-
After chronic stimulation, T cell responses become diminished or non-existent to repeated antigenic encounters.
- Epitope spreading
-
After peptide vaccination, T cells are generated that respond to peptides that were not in the original vaccine formulation, indicating that a secondary round of T cell priming has occurred with antigens taken directly from tumour cells.
- T helper 1
-
(TH1). A helper T cell response that is characterized by interferon-γ (IFNγ) production and stimulation of CD8+ cytotoxic T cells.
- Natural killer (NK) cells
-
A cytotoxic cell of the innate immune system that does not recognize target cells in an antigen-specific manner and kills its targets using similar mechanisms to those of the cytotoxic T lymphocyte.
- Opsonization
-
The phagocytosis of opsonized antigens, most commonly by antibodies and/or complement, from the external environment by dendritic cells or by other antigen-presenting cells.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). The destruction of target cells that are coated with antibodies by innate immune cells expressing Fc receptors, such as natural killer cells, monocytes or macrophages, using cytotoxic substances, such as perforin and granzymes, reactive oxygen species and reactive nitrogen species.
- Complement-dependent cytotoxicity
-
(CDC). The destruction of target cells coated with antibodies by a series of serum proteins that undergo a cascade of enzymatic cleavage and culminate in the formation of pores within the target cell membranes, permeabilizing the cells.
- Memory T cells
-
T cells that have undergone antigenic stimulation at least once and that are capable of rapidly responding to additional antigen encounters.
- Central memory T (TCM) cells
-
Long-lived memory T cells that reside in peripheral blood, lymph nodes and spleen that are capable of undergoing rapid differentiation into effector T cells on antigenic stimulation.
- Adoptive transfer
-
The infusion of cells into animals or patients that have been taken directly from another source or expanded and modified ex vivo.
- Lytic pathway
-
The release of cytotoxic molecules such as perforin and granzymes from cytotoxic T lymphocytes to kill their cognate targets.
- Unfolded protein response
-
A stress response in the endoplasmic reticulum that is triggered by the accumulation of misfolded proteins that initially results in increased protein chaperone synthesis and decreased total protein synthesis in an attempt to remove the misfolded proteins. If misfolded proteins persist, continued activation of this pathway ultimately results in apoptosis.
Rights and permissions
About this article
Cite this article
Vanneman, M., Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12, 237–251 (2012). https://doi.org/10.1038/nrc3237
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3237