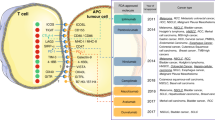
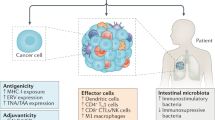
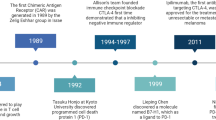
Abstract
The approval of the first immune checkpoint inhibitors provided a paradigm shift for the treatment of malignancies across a broad range of indications. Whereas initially, single-agent immune checkpoint inhibition was used, increasing numbers of patients are now treated with combination immune checkpoint blockade, where non-redundant mechanisms of action of the individual agents generally lead to higher response rates. Furthermore, immune checkpoint therapy has been combined with various other therapeutic modalities, including chemotherapy, radiotherapy and other immunotherapeutics such as vaccines, adoptive cellular therapies, cytokines and others, in an effort to maximize clinical efficacy. Currently, a large number of clinical trials test combination therapies with an immune checkpoint inhibitor as a backbone. However, proceeding without inclusion of broad, if initially exploratory, biomarker investigations may ultimately slow progress, as so far, few combinations have yielded clinical successes based on clinical data alone. Here, we present the rationale for combination therapies and discuss clinical data from clinical trials across the immuno-oncology spectrum. Moreover, we discuss the evolution of biomarker approaches and highlight the potential new directions that comprehensive biomarker studies can yield.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Olson, B., Li, Y., Lin, Y., Liu, E. T. & Patnaik, A. Mouse models for cancer immunotherapy research. Cancer Discov. 8, 1358–1365 (2018).
Diab, A. et al. Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J. Clin. Oncol. 39, 2914–2925 (2021).
Long, G. V. et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20, 1083–1097 (2019).
Freidlin, B. & Korn, E. L. Two-by-two factorial cancer treatment trials: is sufficient attention being paid to possible interactions? J. Natl Cancer Inst. 10.1093/jnci/djx146 (2017).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008).
Talukdar, S. et al. Defining immune infiltrate heterogeneity by immunophenotyping of tumor micro-environment at single cell level: a step towards more effective personalized immunotherapy in ovarian cancer. Gynecol. Oncol. 162, S52 (2021).
Qian, J. & Rankin, E. B. Hypoxia-induced phenotypes that mediate tumor heterogeneity. Adv. Exp. Med. Biol. 1136, 43–55 (2019).
Butterfield, L. H. The society for immunotherapy of cancer biomarkers task force recommendations review. Semin. Cancer Biol. 52, 12–15 (2018).
CLIA. CLIA regulations and federal register documents. Center for Medicare and Medicaid Services https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/CLIA_Regulations_and_Federal_Register_Documents (2023).
Dhainaut, M. et al. Spatial CRISPR genomics identifies regulators of the tumor microenvironment. Cell 185, 1223–1239.e20 (2022).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Eggermont, A. M. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375, 1845–1855 (2016).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378, 1789–1801 (2018).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Robert, C. L. et al. Long-term outcomes in patients (pts) with ipilimumab (ipi)-naïve advanced melanoma in the phase 3. J. Clin. Oncol. 15, 391–402 (2017).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Sharma, P. & Allison, J. P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 20, 75–76 (2020).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Zhu, S. et al. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 14, 156 (2021).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Liu, Y. T. & Sun, Z. J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 11, 5365–5386 (2021).
Spranger, S. et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc. Natl Acad. Sci. USA 113, E7759–E7768 (2016).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Mouw, K. W., Goldberg, M. S., Konstantinopoulos, P. A. & D’Andrea, A. D. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 7, 675–693 (2017).
Ready, N. et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 37, 992–1000 (2019).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
Butterfield, L. H. et al. Multiple antigen-engineered DC vaccines with or without IFNα to promote antitumor immunity in melanoma. J. Immunother. Cancer 7, 113 (2019).
Santos, P. M. et al. Impact of checkpoint blockade on cancer vaccine-activated CD8+ T cell responses. J. Exp. Med. 217, e20191369 (2020).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019).
Dummer, R., Hoeller, C., Gruter, I. P. & Michielin, O. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol. Immunother. 66, 683–695 (2017).
Wang, Y. et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front. Pharmacol. 9, 185 (2018).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Patel, S. A. & Minn, A. J. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity 48, 417–433 (2018).
Gravett, A. M., Trautwein N, Stevanovic, S., Dalgleish, A. G. & Copier, J. Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. Oncoimmunology 7, e1438107 (2018).
Wan, S. et al. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-β signaling in breast cancer cells. PLoS ONE 7, e32542 (2012).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Zhao, J. et al. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 70, 4850–4858 (2010).
Dimeloe, S. et al. Human regulatory T cells lack the cyclophosphamide-extruding transporter ABCB1 and are more susceptible to cyclophosphamide-induced apoptosis. Eur. J. Immunol. 44, 3614–3620 (2014).
Ge, Y. et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol. Immunother. 61, 353–362 (2012).
Buhtoiarov, I. N. et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 132, 226–239 (2011).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Baird, J. D. G. et al. MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo. Providence St. Joseph Health Commons https://digitalcommons.psjhealth.org/sitc2018/ (2018).
Li, J. et al. Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov. 11, 1212–1227 (2021).
Williamson, C. W. et al. Immunotherapy and radiation therapy sequencing: state of the data on timing, efficacy, and safety. Cancer 127, 1553–1567 (2021).
Huard, B., Gaulard, P., Faure, F., Hercend, T. & Triebel, F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics 39, 213–217 (1994).
Kisielow, M., Kisielow, J., Capoferri-Sollami, G. & Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 35, 2081–2088 (2005).
Triebel, F. et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171, 1393–1405 (1990).
Workman, C. J. et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J. Immunol. 182, 1885–1891 (2009).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Kouo, T. et al. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 3, 412–423 (2015).
Wang, J. et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 176, 334–347.e12 (2019).
Xu, F. et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 74, 3418–3428 (2014).
Liang, B. et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 180, 5916–5926 (2008).
Woo, S. R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Ascierto, P. A. et al. Nivolumab and relatlimab in patients with advanced melanoma that had progressed on anti-programmed death-1/programmed death ligand 1 therapy: results from the phase I/IIa RELATIVITY-020 trial. J. Clin. Oncol. 41, 2724–2735 (2023).
Chauvin, J. M. & Zarour, H. M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 8, e000957 (2020).
Rudin, C. M. et al. SKYSCRAPER-02: primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab (atezo) + carboplatin + etoposide (CE) with or without tiragolumab (tira) in patients (pts) with untreated extensive-stage small cell lung cancer (ES-SCLC). J. Clin. Oncol. 40, LBA8507 (2022).
Fourcade, J. et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 10.1172/jci.insight.121157 (2018).
Cho, B. C. et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23, 781–792 (2022).
Dummer, R. et al. Abstract CT002: KEYMAKER-U02 substudy 02C: neoadjuvant pembrolizumab (pembro) + vibostolimab (vibo) or gebasaxturev (geba) or pembro alone followed by adjuvant pembro for stage IIIB-D melanoma. Cancer Res. 83, CT002 (2023).
Solinas, C., De Silva, P., Bron, D., Willard-Gallo, K. & Sangiolo, D. Significance of TIM3 expression in cancer: from biology to the clinic. Semin. Oncol. 46, 372–379 (2019).
Anderson, A. C. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2, 393–398 (2014).
Kato, R. et al. Increased Tim-3+ T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol. Immunother. 67, 1673–1683 (2018).
Han, G., Chen, G., Shen, B. & Li, Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front. Immunol. 4, 449 (2013).
Curigliano, G. et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 27, 3620–3629 (2021).
Chow, A. et al. Tim-4+ cavity-resident macrophages impair anti-tumor CD8+ T cell immunity. Cancer Cell 39, 973–988.e9 (2021).
Milhem, M. et al. O85 durable responses in anti-PD-1 refractory melanoma following intratumoral injection of a toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab. J. Immunother. Cancer 8, A2–A3 (2020).
Yap, T. A. et al. First-in-human phase I/II ICONIC trial of the ICOS agonist vopratelimab alone and with nivolumab: ICOS-high CD4 T-cell populations and predictors of response. Clin. Cancer Res. 28, 3695–3708 (2022).
Byrne, K. T. et al. Neoadjuvant selicrelumab, an agonist CD40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin. Cancer Res. 27, 4574–4586 (2021).
Leblond, M. M. et al. CD40 agonist restores the antitumor efficacy of anti-PD1 therapy in muscle-invasive bladder cancer in an IFN I/II-mediated manner. Cancer Immunol. Res. 8, 1180–1192 (2020).
Ma, H. S. et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol. Res. 7, 428–442 (2019).
Weiss, S. et al. 389 Phase II of CD40 agonistic antibody sotigalimab (APX005M) in combination with nivolumab in subjects with metastatic melanoma with confirmed disease progression on anti-PD-1 therapy. J. Immunother. Cancer 9, A422 (2021).
Kim, T. W. et al. First-in-human phase I study of the OX40 agonist MOXR0916 in patients with advanced solid tumors. Clin. Cancer Res. 28, 3452–3463 (2022).
Ma, Y. et al. Combination of PD-1 inhibitor and OX40 agonist induces tumor rejection and immune memory in mouse models of pancreatic cancer. Gastroenterology 159, 306–319.e12 (2020).
Gutierrez, M. et al. OX40 agonist BMS-986178 alone or in combination with nivolumab and/or ipilimumab in patients with advanced solid tumors. Clin. Cancer Res. 27, 460–472 (2021).
Macedo, N., Miller, D. M., Haq, R. & Kaufman, H. L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 8, e001486 (2020).
Hamid, O., Ismail, R. & Puzanov, I. Intratumoral immunotherapy—update 2019. Oncologist 25, e423–e438 (2020).
Puzanov, I. et al. 433 Talimogene laherparepvec (T-VEC) in combination with ipilimumab (IPI) versus IPI alone for advanced melanoma: 4-year interim analysis of a randomized, open-label, phase 2 trial. J. Immunother. Cancer 8, A263 (2020).
Dummer, R. et al. Randomized phase III trial evaluating spartalizumab plus dabrafenib and trametinib for BRAF V600-mutant unresectable or metastatic melanoma. J. Clin. Oncol. 40, 1428–1438 (2022).
Chesney, J. A. et al. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J. Clin. Oncol. 41, 528–540 (2023).
Middleton, M. et al. 422 An open-label, multicenter, phase 1/2 clinical trial of RP1, an enhanced potency oncolytic HSV, combined with nivolumab: updated results from the skin cancer cohorts. J. Immunother. Cancer 8, A257 (2020).
Milhem, M. M. et al. Updated results from the skin cancer cohorts from an ongoing phase 1/2 multicohort study of RP1, an enhanced potency oncolytic HSV, combined with nivolumab (IGNYTE). J. Clin. Oncol. 40, 9553 (2022).
Najjar, Y. G., Puligandla, M., Lee, S. J. & Kirkwood, J. M. An updated analysis of 4 randomized ECOG trials of high-dose interferon in the adjuvant treatment of melanoma. Cancer 125, 3013–3024 (2019).
Vaishampayan, U. N. et al. Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors: ARTISTRY-1. J. Clin. Oncol. 40, 2500 (2022).
Qiu, Y. et al. Clinical application of cytokines in cancer immunotherapy. Drug. Des. Devel. Ther. 15, 2269–2287 (2021).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Butterfield, L. H. et al. Immune correlates of GM-CSF and melanoma peptide vaccination in a randomized trial for the adjuvant therapy of resected high-risk melanoma (E4697). Clin. Cancer Res. 23, 5034–5043 (2017).
Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin. Cancer Res. 26, 2827–2837 (2020).
Chapuis, A. G. et al. T-cell therapy using interleukin-21-primed cytotoxic T-cell lymphocytes combined with cytotoxic T-cell lymphocyte antigen-4 blockade results in long-term cell persistence and durable tumor regression. J. Clin. Oncol. 34, 3787–3795 (2016).
Kalbasi, A. et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 607, 360–365 (2022).
Melisi, D. et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 9, e002068 (2021).
Escos, A., Risco, A., Alsina-Beauchamp, D. & Cuenda, A. p38γ and p38δ Mitogen activated protein kinases (MAPKs), new stars in the MAPK galaxy. Front. Cell Dev. Biol. 4, 31 (2016).
Liang, X. et al. miR-128 enhances dendritic cell-mediated anti-tumor immunity via targeting of p38. Mol. Med. Rep. 16, 1307–1313 (2017).
Ryan, A. et al. 588 Phase Ib study of the p38 inhibitor ARRY-614 with nivolumab, ipilimumab or nivolumab+ipilimumab in advanced solid tumors. J. Immunother. Cancer 10, A615 (2022).
Clancy-Thompson, E. et al. Peptide vaccination in montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells. Cancer Immunol. Res. 1, 332–339 (2013).
Sarnaik, A. A. et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 39, 2656–2666 (2021).
Jason, C. et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J. Immunother. Cancer 10, e005755 (2022).
O’Malley, D. Phase 2 efficacy and safety of autologous tumor-infiltrating lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor-naïve patients with advanced cancers. J. Immunother. Cancer 10.1136/jitc-2021-SITC2021.492 (2021).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
John, L. B., Kershaw, M. H. & Darcy, P. K. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology 2, e26286 (2013).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Arina, A. & Bronte, V. Myeloid-derived suppressor cell impact on endogenous and adoptively transferred T cells. Curr. Opin. Immunol. 33, 120–125 (2015).
Long, A. H. et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol. Res. 4, 869–880 (2016).
Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 59, 1593–1600 (2010).
Heczey, A. et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 124, 2824–2833 (2014).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies — BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Ribas, A. et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 15, 6267–6276 (2009).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
McNeel, D. G. et al. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-004198 (2022).
Mueller, S. et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J. Clin. Invest. https://doi.org/10.1172/JCI162283 (2022).
Brightman, S. E., Naradikian, M. S., Miller, A. M. & Schoenberger, S. P. Harnessing neoantigen specific CD4 T cells for cancer immunotherapy. J. Leukoc. Biol. 107, 625–633 (2020).
Smith, C. C. et al. Alternative tumour-specific antigens. Nat. Rev. Cancer 19, 465–478 (2019).
Martin, J. D., Fukumura, D., Duda, D. G., Boucher, Y. & Jain, R. K. Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a027094 (2016).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Doedens, A. L. et al. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res. 70, 7465–7475 (2010).
Motz, G. T. & Coukos, G. Deciphering and reversing tumor immune suppression. Immunity 39, 61–73 (2013).
Schiffmann, L. M. et al. A combination of low-dose bevacizumab and imatinib enhances vascular normalisation without inducing extracellular matrix deposition. Br. J. Cancer 116, 600–608 (2017).
Najjar, Y. G. et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight https://doi.org/10.1172/jci.insight.124989 (2019).
Yang, J., Yan, J. & Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 9, 978 (2018).
Roland, C. L. et al. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS ONE 4, e7669 (2009).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aak9679 (2017).
Ott, P. A., Hodi, F. S. & Buchbinder, E. I. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front. Oncol. 5, 202 (2015).
Yasuda, S. et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 172, 500–506 (2013).
Cui, C. et al. Toripalimab plus axitinib versus toripalimab or axitinib alone in patients with treatment-naive unresectable or metastatic mucosal melanoma: interim results from a randomized, controlled, phase II trial. J. Clin. Oncol. 40, 9512 (2022).
Cui, C. et al. A phase 2 clinical trial of neoadjuvant anti-PD-1 Ab (toripalimab) plus axitinib in resectable mucosal melanoma. J. Clin. Oncol. 39, 9512 (2021).
Sheng, X. et al. Axitinib in combination with toripalimab, a humanized immunoglobulin G4 monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: an open-label phase IB trial. J. Clin. Oncol. 37, 2987–2999 (2019).
Wang, X. et al. Apatinib combined with camrelizumab in advanced acral melanoma patients: an open-label, single-arm phase 2 trial. Eur. J. Cancer 182, 57–65 (2023).
Choueiri, T. K. et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 19, 451–460 (2018).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Kimura, T. et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 109, 3993–4002 (2018).
Kato, Y. et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 14, e0212513 (2019).
Taylor, M. H. et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol. 38, 1154–1163 (2020).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384, 1289–1300 (2021).
Makker, V. et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 386, 437–448 (2022).
Arance, A. et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.00221 (2023).
Vano, Y. A. et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 23, 612–624 (2022).
Callahan, M. K. & Chapman, P. B. PD-1 or PD-L1 blockade adds little to combination of BRAF and MEK inhibition in the treatment of BRAF V600-mutated melanoma. J. Clin. Oncol. 40, 1393–1395 (2022).
Wilmott, J. S. et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 18, 1386–1394 (2012).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Schilling, B. & Paschen, A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: neutralization of myeloid-derived suppressor cells. Oncoimmunology 2, e25218 (2013).
Ott, P. A. et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol. Immunother. 62, 811–822 (2013).
Callahan, M. K. et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol. Res. 2, 70–79 (2014).
Hoyer, S. et al. BRAF and MEK inhibitors affect dendritic-cell maturation and T-cell stimulation. Int. J. Mol. Sci. 22, 11951 (2021).
Minor, D. R., Puzanov, I., Callahan, M. K., Hug, B. A. & Hoos, A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment. Cell Melanoma Res. 28, 611–612 (2015).
Ribas, A., Hodi, F. S., Callahan, M., Konto, C. & Wolchok, J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366 (2013).
Ferrucci, P. F. et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J. Immunother. Cancer 8, e001806 (2020).
Storkus, W. J. et al. Dendritic cell vaccines targeting tumor blood vessel antigens in combination with dasatinib induce therapeutic immune responses in patients with checkpoint-refractory advanced melanoma. J. Immunother. Cancer 9, e003675 (2021).
Bollard, C. M. et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 32, 798–808 (2014).
Dummer, R. et al. Atezolizumab, vemurafenib, and cobimetinib in patients with melanoma with CNS metastases (TRICOTEL): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 23, 1145–1155 (2022).
Padron, L. J. et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 28, 1167–1177 (2022).
Farah, A. et al. 587 AMADEUS trial: tumor agnostic pre- and on-treatment biomarkers of response to nivolumab plus ipilimumab correlate with on-treatment tumoral T cell infiltration. J. Immunother. Cancer 10, A614 (2022).
Inman, S. CLDN18.2-directed CAR T-cell therapy effective, safe in gastrointestinal cancers. Cancer Network www.cancernetwork.com/view/cldn18-2-directed-car-t-cell-therapy-effective-safe-in-gastrointestinal-cancers (2022).
Adamik, J. et al. Distinct metabolic states guide maturation of inflammatory and tolerogenic dendritic cells. Nat. Commun. 13, 5184 (2022).
Wolchok, J. D. et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 39, 9506 (2021).
Lebbe, C. et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J. Clin. Oncol. 37, 867–875 (2019).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
Doki, Y. et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386, 449–462 (2022).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Lenz, H. J. et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J. Clin. Oncol. 40, 161–170 (2022).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Finn, R. S. et al. LBA34 — primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.08.031 (2022).
Gutzmer, R. et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395, 1835–1844 (2020).
Eng, C. et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 20, 849–861 (2019).
US National Library of Science. ClinicalTrials.gov clinicaltrials.gov/ct2/show/NCT04511013 (2023).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Merino, D. M. et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J. Immunother. Cancer 8, e000147 (2020).
Vega, D. M. et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann. Oncol. 32, 1626–1636 (2021).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031 (2019).
Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 (2016).
Mok, T. S. K. et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393, 1819–1830 (2019).
Kulangara, K. et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 143, 330–337 (2019).
Muro, K. et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 17, 717–726 (2016).
De Marchi, P. et al. PD-L1 expression by tumor proportion score (TPS) and combined positive score (CPS) are similar in non-small cell lung cancer (NSCLC). J. Clin. Pathol. 74, 735–740 (2021).
Davis, A. A. & Patel, V. G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 7, 278 (2019).
Kim, E. S. et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial. Nat. Med. 28, 939–945 (2022).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
FDA. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (2020).
FDA. FDA grants nivolumab accelerated approval for MSI-H or dMMR colorectal cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-msi-h-or-dmmr-colorectal-cancer (2017).
Goodman, A. M. et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 16, 2598–2608 (2017).
Marcus, L. et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. 27, 4685–4689 (2021).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Valero, C. et al. Response rates to anti-PD-1 immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol. 7, 739–743 (2021).
Yarchoan, M. et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 4, e126908 (2019).
Hellmann, M. D. et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 33, 843–852.e4 (2018).
From the American Association of Neurological Surgeons (AANS)et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 13, 612–632 (2018).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020).
Goodman, A. M., Sokol, E. S., Frampton, G. M., Lippman, S. M. & Kurzrock, R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol. Res. 7, 1570–1573 (2019).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
DiGuardo, M. A. et al. RNA-seq reveals differences in expressed tumor mutation burden in colorectal and endometrial cancers with and without defective DNA-mismatch repair. J. Mol. Diagn. 23, 555–564 (2021).
Overman, M. J. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779 (2018).
Vega, D. M. et al. Changes in circulating tumor DNA reflect clinical benefit across multiple studies of patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. JCO Precis. Oncol. 6, e2100372 (2022).
Davar, D. et al. A phase 2/3 trial in progress on tebentafusp as monotherapy and in combination with pembrolizumab in HLA-A*02:01+ patients with previously treated advanced non-uveal melanoma (TEBE-AM). J. Clin. Oncol. 41, TPS9594 (2023).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Conde, E. et al. Epitope spreading driven by the joint action of CART cells and pharmacological STING stimulation counteracts tumor escape via antigen-loss variants. J. Immunother. Cancer 9, e003351 (2021).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Ruffin, A. T. et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 12, 3349 (2021).
Campbell, M. T. et al. Pilot study of tremelimumab with and without cryoablation in patients with metastatic renal cell carcinoma. Nat. Commun. 12, 6375 (2021).
Taube, J. M. et al. Multi-institutional TSA-amplified Multiplexed Immunofluorescence Reproducibility Evaluation (MITRE) study. J. Immunother. Cancer 9, e002197 (2021).
Black, S. et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 16, 3802–3835 (2021).
Hickey, J. W. et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nat. Methods 19, 284–295 (2022).
Zhang, W. et al. Identification of cell types in multiplexed in situ images by combining protein expression and spatial information using CELESTA. Nat. Methods 19, 759–769 (2022).
Acknowledgements
This work was supported by the Parker Institute for Cancer Immunotherapy (L.H.B.). This work was supported by the University of Pittsburgh UPMC Hillman Cancer Center Award P30CA04904 (Y.G.N.). The authors thank D. Gilmartin for assistance with the references in this paper.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
L.H.B. declares the following unrelated advisory activities: Calidi Scientific and Medical Advisory Board, 6 April 2017–2023; KaliVir, Scientific Advisory Board, 2018–2023; Torque Therapeutics, Scientific Advisory Board, 2018–2020; Khloris, Scientific Advisory Board, 2019–2023; Pyxis, Scientific Advisory Board, 2019–2023; CytomX, Scientific Advisory Board, 2019–2023; DCprime, Scientific Advisory Board meeting, November 2020; RAPT, Scientific Advisory Board, 2020–2023; Takeda, scientific advisor, 2020–2023; EnaraBio scientific adviser, February 2021; Federation Bio, scientific adviser, 2022; and Pfizer, scientific adviser, 2022. Current contact: lisa.butterfield@merck.com. Y.G.N. declares the following unrelated advisory activities: Scientific Advisory Board/consulting: Merck, InterVenn Bio, Novartis, BMS, Pfizer and Immunocore. Non-continuing education honoraria: Immunocore. Travel cost/accommodations: Istari Oncology. Research funding (to institution): Merck, Pfizer, BMS and Replimune.
Peer review
Peer review information
Nature Reviews Immunology thanks Taha Merghoub, Brent Hanks and Dirk Jaeger for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Abscopal effect
-
The observation that a local treatment delivered to one anatomical site (for example, the tumour) leads to an effect at a distant anatomical site (for example, a metastatic tumour deposit), suggesting circulating systemic immunity.
- Antigenic breadth
-
The number of different tumour antigens (or epitopes therein) that the immune system reacts to.
- Epitope spreading
-
The phenomenon identified in autoimmunity in which an immune response to one tissue antigen leads to cell death, additional antigen release in inflammatory conditions and subsequent rounds of immune response to new antigens.
- Hypothesis-generating biomarkers
-
Broad testing of immune response that is not directed towards a specific scientific hypothesis, but which instead is designed to provide data to be mined to identify unanticipated hypotheses.
- Immune monitoring of patients
-
Immune response testing performed in clinical trials on patient blood and other tissue samples to determine the effects of a therapy.
- ImmunoPET
-
PET using labels that detect immune cells (for example, CD8+ T cells).
- Immunophenotype
-
The observable characteristics that relate to the immune system and immune reactivity.
- M1-polarized
-
Myeloid cells (macrophages) whose functions are aligned with antitumour functions, so called in reference to the ‘type 1’ T cell paradigm.
- M2-polarized
-
Myeloid cells (macrophages) whose functions are in opposition to antitumour functions, so called in reference to the ‘type 2’ T cell paradigm.
- Tertiary lymphoid structures
-
Organized immune structures that can be detected in different diseased tissues that resemble a secondary lymphoid structure containing T and B cells, antibodies and antigen-presenting cells.
- T cell engagers
-
Chimeric molecules, commonly with antibody structures, which simultaneously bind to the CD3 molecule on the T cell surface and a cell-surface tumour protein to connect T cells with tumour cells directly.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Butterfield, L.H., Najjar, Y.G. Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nat Rev Immunol (2023). https://doi.org/10.1038/s41577-023-00973-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-023-00973-8