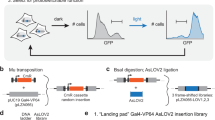
Abstract
Light-triggered gene expression systems offer an unprecedented spatiotemporal resolution that cannot be achieved with classical chemically inducible genetic tools. Here we describe a protocol for red light–responsive gene expression in mammalian cells. This system can be toggled between stable ON and OFF states by short pulses of red and far-red light, respectively. In the protocol, CHO-K1 cells are transfected to allow red light–inducible expression of the secreted alkaline phosphatase (SEAP) reporter, and gene expression is tuned by illumination with light of increasing wavelengths. As a starting point for elaborate red light–responsive gene expression, we outline the reversible activation of gene expression and describe how a spatial pattern can be 'printed' on a monolayer of cells by using a photomask. The core protocol requires only 4 d from seeding of the cells to reporter quantification, and other than light-emitting diode (LED) illumination boxes no elaborate equipment is required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weber, W. & Fussenegger, M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 13, 21–35 (2012).
Horner, M. & Weber, W. Molecular switches in animal cells. FEBS Lett. 586, 2084–2096 (2012).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Muller, K. & Weber, W. Optogenetic tools for mammalian systems. Mol. Biosyst. 9, 596–608 (2013).
Toettcher, J.E., Voigt, C.A., Weiner, O.D. & Lim, W.A. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat. Methods 8, 35–38 (2011).
Levskaya, A., Weiner, O.D., Lim, W.A. & Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Schroder-Lang, S. et al. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 (2007).
Wu, Y.I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Kennedy, M.J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Zhou, X.X., Chung, H.K., Lam, A.J. & Lin, M.Z. Optical control of protein activity by fluorescent protein domains. Science 338, 810–814 (2012).
Muller, K. et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 41, e77 (2013).
Muller, K. et al. Synthesis of phycocyanobilin in mammalian cells. Chem. Commun. 49, 8970–8972 (2013).
Kicheva, A., Cohen, M. & Briscoe, J. Developmental pattern formation: insights from physics and biology. Science 338, 210–212 (2012).
Davidson, E.H. Emerging properties of animal gene regulatory networks. Nature 468, 911–920 (2010).
Muller, K. et al. Multi-chromatic control of mammalian gene expression and signaling. Nucleic Acids Res. 41, e124 (2013).
Ye, H.F., Daoud-El Baba, M., Peng, R.W. & Fussenegger, M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568 (2011).
Wang, X., Chen, X. & Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 9, 266–269 (2012).
Konermann, S. et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013).
Crefcoeur, R.P., Yin, R., Ulm, R. & Halazonetis, T.D. Ultraviolet-B–mediated induction of protein-protein interactions in mammalian cells. Nat. Commun. 4, 1779 (2013).
Wang, R.J. Effect of room fluorescent light on the deterioration of tissue culture medium. In Vitro 12, 19–22 (1976).
Cadet, J., Mouret, S., Ravanat, J.L. & Douki, T. Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem. Photobiol. 88, 1048–1065 (2012).
Pattison, D.I., Rahmanto, A.S. & Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 11, 38–53 (2012).
Fussenegger, M., Moser, S., Mazur, X. & Bailey, J.E. Autoregulated multicistronic expression vectors provide one-step cloning of regulated product gene expression in mammalian cells. Biotechnol. Prog. 13, 733–740 (1997).
Cole, W.J., Chapman, D.J. & Siegelman, H.W. The structure and properties of phycocyanobilin and related bilatrienes. Biochemistry 7, 2929–2935 (1968).
Pratt, L.H. & Butler, W.L. Phytochrome conversion by ultraviolet light. Photochem. Photobiol. 11, 503–509 (1970).
Kutner, R.H., Zhang, X.Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1–based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009).
Acknowledgements
This work was supported by contract research 'Internationale Spitzenforschung II' of the Baden-Württemberg Stiftung (P-LS-SPII/2), by the European Research Council (ERC) under the European Community′s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 259043-CompBioMat, and by the Excellence Initiative of the German Federal and State Governments (EXC 294). We thank J. Schmidt, D. Schächtele and J. Meßmer (University of Freiburg) for designing and constructing the illumination boxes.
Author information
Authors and Affiliations
Contributions
K.M. designed and performed the experiments, analyzed the data and wrote the paper; M.D.Z. and W.W. designed the experiments, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Patent applications have been filed (European Patent Office, World Intellectual Property Organization) by the Baden-Württemberg Stiftung covering this technology, for which K.M., M.D.Z. and W.W. are inventors.
Integrated supplementary information
Supplementary Figure 1 Oxidation of PCB in cell culture medium.
(A) Change in the absorbance spectrum. 15 μM PCB was added to cell culture medium and incubated at 37 °C, 5% CO2. At the indicated points in time after PCB addition, samples were removed and the absorbance spectrum between 300 nm and 700 nm was measured. (B) Color change of the PCB-supplemented medium-samples from (A).
Supplementary information
Oxidation of PCB in cell culture medium.
(A) Change in the absorbance spectrum. 15 μM PCB was added to cell culture medium and incubated at 37 °C, 5% CO2. At the indicated points in time after PCB addition, samples were removed and the absorbance spectrum between 300 nm and 700 nm was measured. (B) Color change of the PCB-supplemented medium-samples from (A). (PDF 240 kb)
Rights and permissions
About this article
Cite this article
Müller, K., Zurbriggen, M. & Weber, W. Control of gene expression using a red- and far-red light–responsive bi-stable toggle switch. Nat Protoc 9, 622–632 (2014). https://doi.org/10.1038/nprot.2014.038
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.038
This article is cited by
-
Towards translational optogenetics
Nature Biomedical Engineering (2022)
-
Optogenetic control of gene expression in plants in the presence of ambient white light
Nature Methods (2020)
-
Deconstructing and repurposing the light-regulated interplay between Arabidopsis phytochromes and interacting factors
Communications Biology (2019)
-
Optogenetic control of integrin-matrix interaction
Communications Biology (2019)
-
Dual-controlled optogenetic system for the rapid down-regulation of protein levels in mammalian cells
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.