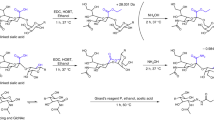
Abstract
Glycosaminoglycans (GAGs) have proven to be very difficult to analyze and characterize because of their high negative charge density, polydispersity and sequence heterogeneity. As the specificity of the interactions between GAGs and proteins results from the structure of these polysaccharides, an understanding of GAG structure is essential for developing a structure–activity relationship. Electrospray ionization (ESI) mass spectrometry (MS) is particularly promising for the analysis of oligosaccharides chemically or enzymatically generated by GAGs because of its relatively soft ionization capacity. Furthermore, on-line high-performance liquid chromatography (HPLC)-MS greatly enhances the characterization of complex mixtures of GAG-derived oligosaccharides, providing important structural information and affording their disaccharide composition. A detailed protocol for producing oligosaccharides from various GAGs, using controlled, specific enzymatic or chemical depolymerization, is presented, together with their HPLC separation, using volatile reversed-phase ion-pairing reagents and on-line ESI-MS structural identification. This analysis provides an oligosaccharide map together with sequence information from a reading frame beginning at the nonreducing end of the GAG chains. The preparation of oligosaccharides can be carried out in 10 h, with subsequent HPLC analysis in 1–2 h and HPLC-MS analysis taking another 2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Bielik, A.M. & Zaia, J. Historical overview of glycoanalysis. Methods Mol. Biol. 600, 9–30 (2010).
Sasisekharan, R. et al. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu. Rev. Biomed. Eng. 8, 181–231 (2006).
Ly, M. et al. Proteoglycanomics: Recent progress and future challenges. OMICS (in press).
Dennis, J.W., Granovsky, M. & Warren, C.E. Protein glycosylation in development and disease. Bioessays 21, 412–421 (1999).
Morgan, M.R., Humphries, M.J. & Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell. Biol. 8, 957–969 (2007).
Gama, C.I. & Hsieh-Wilson, L.C. Chemical approaches to deciphering the glycosaminoglycan code. Curr. Opin. Chem. Biol. 9, 609–619 (2005).
Capila, I. & Linhardt, R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 41, 391–412 (2002).
Raman, R. et al. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem. Biol. 12, 267–277 (2005).
Yamada, S. & Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug. Discov. Technol. 5, 289–301 (2008).
Yip, G.W. et al. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 5, 2139–2148 (2006).
Lee, J.S. & Chien, C.B. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat. Rev. Genet. 5, 923–935 (2004).
Casu, B. et al. Structural and conformational aspects of the anticoagulant and anti-thrombotic activity of heparin and dermatan sulfate. Curr. Pharm. Des. 10, 939–949 (2004).
Shriver, Z. et al. Glycomics: a pathway to a class of new and improved therapeutics. Nat. Rev. Drug Discov. 3, 863–873 (2004).
Linhardt, R.J. & Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 25 (Suppl 3): 5–16 (1999).
Zhang, Z. et al. Solution structure of chemoenzymatic synthesized heparin and its precursors. J. Am. Chem. Soc. 130, 12998–13007 (2008).
Lindahl, U. et al. Generation of 'neoheparin' from E. coli K5 capsular polysaccharide. J. Med. Chem. 48, 349–352 (2005).
Kuberan, B. et al. Chemoenzymatic synthesis of classical and non-classical anticoagulant heparan sulfate polysaccharides. J. Biol. Chem. 278, 52613–52621 (2003).
Naggi, A. et al. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin. Thromb. Hemost. 27, 437–443 (2001).
Rodriguez, M.L., Jann, B. & Jann, K. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose-containing polysaccharide with a chondroitin backbone. Eur. J. Biochem. 177, 117–124 (1988).
Griffiths, G., Barrett, B., Cook, N. & Roberts, I.S. Biosynthesis of the Escherichia coli K5 capsular polysaccharide. Biochem. Soc. Trans. 27, 507–512 (1999).
DeAngelis, P.L., Gunay, N.S., Toida, T., Mao, W.J. & Linhardt, R.J. Identification of the capsular polysaccharides of type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively. Carbohydr. Res. 337, 1547–1552 (2002).
Martin, J.G. et al. Toward an artificial Golgi: redesigning the biological activities of heparan sulfate on a digital microfluidic chip. J. Am. Chem. Soc. 131, 11041–11048 (2009).
Laremore, T.N., Zhang, F., Dordick, J.S., Liu, J. & Linhardt, R.J. Recent progress and applications in glycosaminoglycan and heparin research. Curr. Opin. Chem. Biol. 13, 633–640 (2009).
Silbert, J.E. & Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 54, 177–186 (2002).
Esko, J.D. & Selleck, S.B. Order out of chaos: assembly and ligand binding in heparin sulfate. Annu. Rev. Biochem. 71, 435–471 (2002).
Thanawiroon, C. et al. Liquid chromatography/mass spectrometry sequencing approach for highly sulfated heparin-derived oligosaccharides. J. Biol. Chem. 279, 2608–2615 (2004).
Dell, A., Reason, A.J., Khoo, K.H., Panico, M., McDowell, R.A. & Morris, H.R. Mass spectrometry of carbohydrate-containing biopolymers. Methods Enzymol. 230, 108–132 (1994).
Prabhakar, V. et al. The structural elucidation of glycosaminoglycans. Methods Mol. Biol. 534, 147–156 (2009).
Volpi, N. et al. Capillary electrophoresis of complex natural polysaccharides. Electrophoresis 29, 3095–3106 (2008).
Raman, R. et al. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods 2, 817–824 (2005).
Bindila, L. & Peter-Katalinić, J. Chip-mass spectrometry for glycomic studies. Mass Spectrom. Rev. 28, 223–253 (2009).
Conrad, H.E. Nitrous acid degradation of glycosaminoglycans. In Analysis of Glycoconjugates. (ed. Varki, A.) Chapter 17: Unit 17.22A (Wiley Interscience, Boston, 2001).
Linhardt, R.J. Analysis of glycosaminoglycans with polysaccharide lyases. In Analysis of Glycoconjugates. (ed. Varki, A.) Chapter 17: Unit 17.13.17 (Wiley Interscience, Boston, 2001).
Imanari, T. et al. High-performance liquid chromatographic analysis of glycosaminoglycan-derived oligosaccharides. J. Chromatogr. A 720, 275–293 (1996).
Storm, T. et al. Use of volatile amines as ion-pairing agents for the high-performance liquid chromatographic-tandem mass spectrometric determination of aromatic sulfonates in industrial wastewater. J. Chromatogr. A 854, 175–185 (1999).
Witters, E. et al. Ion-pair liquid chromatography-electrospray mass spectrometry for the analysis of cyclic nucleotides. J. Chromatogr. B 694, 55–63 (1997).
Heinegard, D. & Sommarin, Y. Isolation and characterization of proteoglycans. Methods Enzymol. 144, 319–373 (1987).
Volpi, N. (ed.) Chondroitin Sulfate: Structure, Role And Pharmacological Activity (Academic Press, New York, 2006).
Beaty, N.B. & Mello, R.J. Extracellular mammalian polysaccharides: glycosaminoglycans and proteoglycans. J. Chromatogr. 418, 187–222 (1987).
Roden, L. et al. Isolation and characterization of connective tissue polysaccharides. Methods Enzymol. 28, 73–140 (1972).
Zhang, F. et al. Microscale isolation and analysis of heparin from plasma using an anion exchange column. Anal. Biochem. 353, 284–286 (2006).
Volpi, N. et al. Mass spectrometry for the characterization of unsulfated chondroitin oligosaccharides from 2-mers to 16-mers. Comparison with hyaluronic acid oligomers. Rapid Commun. Mass Spectrom. 22, 3526–3530 (2008).
Volpi, N. Mass spectrometry characterization of Escherichia coli K4 oligosaccharides from 2-mers to more than 20-mers. Rapid Commun. Mass Spectrom. 21, 3459–3466 (2007).
Volpi, N. Chondroitin C lyase [4.2.2.] is unable to cleave fructosylated sequences inside the partially fructosylated Escherichia coli K4 polymer. Glycoconj. J. 25, 451–457 (2008).
Thanawiroon, C. & Linhardt, R.J. Separation of a complex mixture of heparin-derived oligosaccharides using reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1014, 215–223 (2003).
Volpi, N. & Maccari, F. Structural characterization and antithrombin activity of dermatan sulfate purified from marine clam Scapharca inaequivalvis. Glycobiology 19, 356–367 (2009).
Volpi, N. On-line HPLC/ESI-MS separation and characterization of hyaluronan oligosaccharides from 2-mers to 40-mers. Anal. Chem. 79, 6390–6397 (2007).
Zhang, Z. et al. Tandem MS can distinguish hyaluronic acid from N-acetylheparosan. J. Am. Soc. Mass Spectrom. 19, 82–90 (2008).
Midura, R.J. et al. Nonreducing end structures of chondroitin sulfate chains on aggrecan isolated from Swarm rat chondrosarcoma cultures. J. Biol. Chem. 270, 8009–8015 (1995).
Zhang, Z. et al. Liquid chromatography–mass spectrometry to study chondroitin lyase action pattern. Anal. Biochem. 385, 57–64 (2009).
Volpi, N. & Maccari, F. LC separation and online MS characterization of saturated and unsaturated alginic acid oligomers. Chromatographia 69, 813–819 (2009).
Domon, B. & Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 5, 397–409 (1988).
Bitter, T. & Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334 (1962).
Acknowledgements
This work was supported in part through grants from the US National Institutes of Health (GM38060, HL096972, HL101721 and GM090127) to R.J.L.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. R.J.L. designed and developed the RPIP-HPLC-ESI-MS. R.J.L. and N.V. applied this methodology to the structural study of various complex natural polymers with respect to bacterial polysaccharides.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Volpi, N., Linhardt, R. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nat Protoc 5, 993–1004 (2010). https://doi.org/10.1038/nprot.2010.48
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.48
This article is cited by
-
Development and Validation of UHPLC-MS/MS Method for Quantifying of Agarotriose: An Application for Pharmacokinetic, Tissue Distribution, and Excretion Studies in Rats
Journal of Ocean University of China (2023)
-
A mutant-cell library for systematic analysis of heparan sulfate structure–function relationships
Nature Methods (2018)
-
Efficient recovery of glycosaminoglycan oligosaccharides from polyacrylamide gel electrophoresis combined with mass spectrometry analysis
Analytical and Bioanalytical Chemistry (2017)
-
Glycosaminoglycanomics: where we are
Glycoconjugate Journal (2017)
-
Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone–labeled disaccharides with LC-fluorescence and LC-MS detection
Nature Protocols (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.