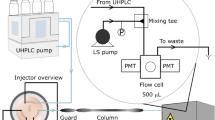
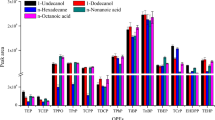
Abstract
Aqueous micropollutants such as estradiol can have a large environmental impact—even at low concentrations. Part of understanding this impact involves determining the extent to which the micropollutants interact with macromolecules in water. In environmental samples, relevant macromolecules to which micropollutants bind are referred to as dissolved organic matter, and the most common examples of these in freshwater and coastal seawater are fulvic and humic acids. In living organisms, the most common macromolecules that affect bioavailability of a drug (or toxin) are proteins such as albumin. Using [2, 4, 6, 7 – 3H]estradiol as an example compound, this protocol uses solid-phase microextraction and scintillation detection as analytical tools to quantify the amount of radiolabeled micropollutant available in solution. The measured free concentration after exposure to various concentrations of macromolecule (dissolved organic matter or protein) or micropollutant is used to determine the partition coefficient in the case of micropollutant–macromolecule interactions. The calibration and preparatory studies take at least 8 d, and the steps to determine the partition coefficient can be completed within 3 d. The protocol could be modified such that nonlabeled compounds are studied; instead of detection of activity by a liquid scintillation counter (LSC), the compounds can be quantified using gas chromatography–mass spectrometry (GC–MS) or liquid chromatography (LC)–MS(/MS).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schwarzenbach, R.P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006).
Schriks, M., Heringa, M.B., van der Kooi, M.M.E., de Voogt, P. & van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 44, 461–476 (2010).
Kolpin, D.W. et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 36, 1202–1211 (2002).
Braga, O., Smythe, G.A., Schäfer, A.I. & Feitz, A.J. Steroid estrogens in ocean sediments. Chemosphere 61, 827–833 (2005).
Trasande, L. et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 100, 1245–1255 (2015).
Jobling, S., Nolan, M., Tyler, C., Brighty, G. & Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 32, 2498–2506 (1998).
Reinthaler, F.F. et al. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 37, 1685–1690 (2003).
Belpaire, C., Geeraerts, C., Roosens, L., Neels, H. & Covaci, A. What can we learn from monitoring PCBs in the European eel? A Belgian experience. Environ. Int. 37, 354–364 (2011).
Sumpter, J.P. The ecotoxicology of hormonally active micropollutants. Water Sci. Technol. 57, 125–130 (2008).
Sharp, R.M. & Skakkebaek, N.E. Male reproductive disorders and the role of endocrine disruption: advances in understanding and identification of areas for future research. Pure Appl. Chem. 75, 2023–2038 (2003).
Chiou, C., Malcolm, R.L., Brinton, T.I. & Kile, D.E. Water solubility enhancement of some organic pollutants and pesticides by dissolved humic and fulvic acids. Environ. Sci. Technol. 20, 502–508 (1986).
Schwarzenbach, R.P., Gschwend, P.W. & Imboden, D.M. Environmental Organic Chemistry 2nd edn (John Wiley and Sons, 2003).
Karickhoff, S.W. Organic pollutant sorption in aquatic systems. J. Hydraul. Eng. 110, 707–735 (1984).
Backhus, D.A. & Gschwend, P.W. Fluorescent polycyclic aromatic hydrocarbons as probes for studying the impact of colloids on pollutant transport in groundwater. Environ. Sci. Technol. 24, 1214–1223 (1990).
Perminova, I.A. et al. Quantification and prediction of the detoxifying properties of humic substances related to their chemical binding to polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 35, 3841–3848 (2001).
Landrum, P.F. Bioavailability and toxicokinetics of polycyclic aromatic hydrocarbons sorbed to sediments for the amphipod Pontoporeia hoyi. Environ. Sci. Technol. 23, 588–595 (1989).
Akkanen, J. & Kukkonen, J.V.K. Measuring the bioavailability of two hydrophobic organic compounds in the presence of dissolved organic matter. Environ. Toxicol. Chem. 22, 518–524 (2003).
Lindsey, M.E. & Tarr, M.A. Inhibition of hydroxyl radical reaction with aromatics by dissolved natural organic matter. Environ. Sci. Technol. 34, 444–449 (2000).
Herbert, B.E., Bertsch, P.M. & Novak, J.M. Pyrene sorption by water-soluble organic carbon. Environ. Sci. Technol. 27, 398–403 (1993).
Ballard, B.D. & MacKay, A.A. Estimating the removal of anthropogenic organic chemicals from raw drinking water by coagulation flocculation. J. Environ. Eng. 131, 108–118 (2005).
Ng, H.Y. & Elimelech, M. Influence of colloidal fouling on rejection of trace organic contaminants by reverse osmosis. J. Membr. Sci. 244, 215–226 (2004).
Hu, J.Y., Jin, X., & Ong, S.L. Rejection of estrone by nanofiltration: influence of solution chemistry. J. Membr. Sci. 302, 188–196 (2007).
Mott, H.V. Association of hydrophobic organic contaminants with soluble organic matter: evaluation of the database of Kdoc values. Environ. Res. 6, 577–593 (2002).
Jermann, D., Pronk, W., Boller, M. & Schafer, A.I. The role of NOM fouling for the retention of estradiol and ibuprofen during ultrafiltration. J. Membr. Sci. 329, 75–84 (2009).
Baronti, C. et al. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ. Sci. Technol. 34, 5059–5066 (2000).
Carballa, M. et al. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 38, 2918–2926 (2004).
Heringa, M.B., Pastor, D., Algra, J., Vaes, W.H.J. & Hermens, J.L.M. Negligible depletion solid-phase microextraction with radiolabeled analytes to study free concentrations and protein binding: an example with [3H]estradiol. Anal. Chem. 74, 5993–5997 (2002).
Neale, P.A., Escher, B.I. & Schafer, A.I. Quantification of solute-solute interactions using negligible-depletion solid phase microextraction: measuring the affinity of estradiol for bulk organic matter. Environ. Sci. Technol. 42, 2886–2892 (2008).
Wang, W., Delgado-Moreno, L., Ye, Q. & Gan, J. Improved measurements of partition coefficients for polybrominated diphenyl ethers. Environ. Sci. Technol. 45, 1521–1527 (2011).
Xie, M., Yang, Z.-Y., Bao, L.-J. & Zeng, E.Y. Equilibrium and kinetic solid-phase microextraction determination of the partition coefficients between polychlorinated biphenyl congeners and dissolved humic acid. J. Chromatogr. A 1216, 4553–4559 (2009).
Bandow, N., Altenburger, R. & Brack, W. Application of nd-SPME to determine freely dissolved concentrations in the presence of green algae and algae-water partition coefficients. Chemosphere 79, 1070–1076 (2010).
Prosen, H., Fingler, S., Zupančič-Kralj, L. & Drevenkar, V. Partitioning of selected environmental pollutants into organic matter as determined by solid-phase microextraction. Chemosphere 66, 1580–1589 (2007).
Urrestarazu Ramos, E., Meijer, S.N., Vaes, W.H.J., Verhaar, H.J.M. & Hermens, J.L.M. Using solid-phase microextraction to determine partition coefficients to humic acids and bioavailable concentrations of hydrophobic chemicals. Environ. Sci. Technol. 32, 3430–3435 (1998).
Lang, S.-C. et al. Equilibrium passive sampling as a tool to study polycyclic aromatic hydrocarbons in Baltic Sea sediment pore-water systems. Mar. Pollut. Bull. 101, 296–303 (2015).
Neale, P.A., Escher, B. & Schafer, A.I. Interaction of steroid hormones with organic matter at environmental concentrations as a function of pH. Sci. Total Environ. 407, 1164–1173 (2009).
Poerschmann, J., Kopinke, F.-D. & Pawliszyn, J. Solid phase microextraction to study the sorption of organotin compounds onto particulate and dissolved humic organic matter. Environ. Sci. Technol. 31, 3629–3636 (1997).
Poerschmann, J., Zhangh, Z., Kopinke, F.-D. & Pawliszyn, J. Solid phase microextraction for determining the distribution of chemicals in aqueous matrices. Anal. Chem. 69, 579–600 (1997).
Risticevic, S., Lord, H., Gorecki, T., Arthur, C.L. & Pawliszyn, J. Protocol for solid-phase microextraction method development. Nat. Protoc. 5, 122–139 (2010).
Carter, C.W. & Suffet, I.H. Binding of DDT to dissolved humic substances. Environ. Sci. Technol. 16, 735–740 (1982).
Danielsen, K.M., Chin, Y.-P., Buterbaugh, J.S., Gustafson, T.L. & Traina, S.J. Solubility enhancement and fluorescence quenching of pyrene by humic substances: the effect of dissolved oxygen on quenching processes. Environ. Sci. Technol. 29, 2162–2165 (1995).
McCarthy, J.F. & Jimenez, B.D. Interactions between polycyclic aromatic hydrocarbons and dissolved humic material: binding and dissociation. Environ. Sci. Technol. 19, 1072–1076 (1985).
Landrum, P.F., Nihart, S.R., Eadie, B.J. & Gardner, W.S. Reverse-phase separation method for determining pollutant binding to aldrich humic acid and dissolved organic carbon of natural waters. Environ. Sci. Technol. 18, 187–192 (1984).
Fan, G.T., Burnison, B.K. & Solomon, K.R. The partitioning of fenvalerate to natural dissolved organic matter. Water Res. 31, 2429–2434 (1997).
Gauthier, T.D., Shane, E.C., Guerin, W.F., Seitz, W.R. & Grant, C.L. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ. Sci. Technol. 20, 1162–1166 (1986).
Tiller, C.L. & Jones, K.D. Effects of dissolved oxygen and light exposure on determination of KOC values for PAHs using fluorescence quenching. Environ. Sci. Technol. 31, 424–429 (1997).
Mott, H.V. Association of hydrophobic organic contaminants with soluble organic matter: evaluation of the database of Kdoc values. Adv. Environ. Res. 6, 577–593 (2002).
Akkanen, J., Tuikka, A. & Kukkonen, J.V.K. Comparative sorption and desorption of benzo[a]pyrene and 3,4,3′,4′-tetrachlorobiphenyl in natural lake water containing dissolved organic matter. Environ. Sci. Technol. 39, 7529–7534 (2005).
Neale, P.A. Influence of Solute-Solute Interactions on Membrane Filtration. PhD thesis, University of Edinburgh (2009).
Vuckovic, D., Zhang, X., Cudjoe, E. & Pawliszyn, J. Solid-phase microextraction in bioanalysis: new devices and directions. J. Chromatogr. A 1217, 4041–4060 (2010).
Vaes, W.H.J. et al. Solid phase microextraction as a tool to determine membrane/water partition coefficients and bioavailable concentrations in in vitro systems. Chem. Res. Toxicol. 10, 1067–1072 (1997).
Heringa, M.B. et al. Toward more useful in vitro toxicity data with measured free concentrations. Environ. Sci. Technol. 38, 6263–6270 (2004).
Chang, H.-S., Choo, K.-H., Lee, B. & Choi, S.-J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 172, 1–12 (2009).
Heringa, M.B. & Hermens, J.L.M. Measurement of free concentrations using negligible depletion-solid phase microextraction (nd-SPME). Trends Anal. Chem. 22, 575–587 (2003).
Mackenzie, K., Georgi, A., Kumke, M. & Kopinke, F.-D. Sorption of pyrene to dissolved humic substances and related model polymers. 2. Solid-phase microextraction (SPME) and fluorescence quenching technique (FQT) as analytical methods. Environ. Sci. Technol. 36, 4403–4409 (2002).
ter Laak, T.L., Durjava, M., Struijs, J. & Hermens, J.L.M. Solid phase dosing and sampling technique to determine partition coefficients of hydrophobic chemicals in complex matrixes. Environ. Sci. Technol. 39, 3736–3742 (2005).
Endo, S., Droge, S.T.J. & Goss, K.-U. Polyparameter linear free energy models for polyacrylate fiberwater partition coefficients to evaluate the efficiency of solid-phase microextraction. Anal. Chem. 83, 1394–1400 (2011).
Boyd-Boland, A.A. et al. New solvent-free sample preparation techniques based on fiber and polymer technologies. Environ. Sci. Technol. 28, 569A–574A (1994).
Gjessing, E.T., Egeberg, P.K. & Hakedal, J. Natural organic matter in drinking water-the ′NOM-typing project′, background and basic characteristics of original water sample and NOM isolates. Environ. Int. 25, 145–159 (1999).
Haftka, J.J.H., Govers, H.A.J. & Parsons, J.R. Influence of temperature and origin of dissolved organic matter on the partitioning behavior of polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 17, 1070–1079 (2009).
Frimmel, F.H. & Abbt-Braun, G. Basic characterization of reference NOM from Central Europe-similarities and differences. Environ. Int. 25, 191–207 (1999).
Yuan, H., Pawliszyn, J., Ranatunga, R. & Carr, P.W. Determination of equilibrium constant of alkylbenzenes binding to bovine serum albumin by solid phase microextraction. Analyst 124, 1443–1448 (1999).
Musteata, F.M., Pawliszyn, J., Qian, M.G., Wu, J.-T. & Miwa, G.T. Determination of drug plasma protein binding by solid phase microextraction. J. Pharm. Sci. 95, 1712–1722 (2006).
Tan, Y. & Siebert, K.J. Modeling bovine serum albumin binding of flavor compounds (alcohols, aldehydes, esters, and ketones) as a function of molecular properties. J. Food Sci. 73, S56–S63 (2008).
Böhm, L. & Düring, R.-A. Partitioning of polycyclic musk compounds in soil and aquatic environment—experimental determination of KDOC. J. Soils Sediments 10, 708–713 (2010).
SCR PhysProp Database http://www.srcinc.com/what-we-do/environmental/scientific-databases.html.
Lord, H.L., Grant, R.P., Walles, M., Incledon, B., Fahie, B. & Pawliszyn, J.B. Development and evaluation of a solid-phase microextraction probe for in vivo pharmacokinetic studies. Anal. Chem. 75, 5103–5115 (2003).
Worch, E. Eine neue Gleichung zur Berechnung von Diffusionskoeffizienten geloster Stoffe. Vom Wasser. 81, 289–297 (1993).
Lai, K., Johnson, K., Scrimshaw, M. & Lester, J. Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environ. Sci. Technol. 34, 3890–3894 (2000).
Lide, D. Crc Handbook of Chemistry and Physics (CRC Press/Taylor and Francis, 2008).
Hansch, C., Leo, A. & Hoekman, D. Exploring Qsar: Hydrophobic, Electronic, and Steric Constants (Washington, DC: American Chemical Society, 1995).
Nghiem, L.D., Schäfer, A.I. & Elimelech, M. Removal of natural hormones by nanofiltration membranes: measurement, modelling and mechanisms. Environ. Sci. Technol. 38, 1888–1896 (2004).
Kwon, J.-H., Liljestrand, H.M. & Katz, L.E. Partitioning of moderately hydrophobic endocrine disrupters between water and synthetic membrane vesicles. Environ. Toxicol. Chem. 25, 1984–1992 (2006).
Acknowledgements
H.L.B. acknowledges her Royal Academy of Engineering/EPSRC Fellowship for method development during her study at the University of Edinburgh, UK. A.I.S. acknowledges funding from the Helmholtz Association. The authors thank B. Escher for collaboration with A.I.S. and P. Neale while at EAWAG, Switzerland, that resulted in this method. The authors also thank J. Hermens for useful comments on the SPME technique and F. Schramm for proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
H.L.B. wrote the manuscript, with significant contributions from M.H., who was involved in the SPME method development, and A.I.S., who worked with B. Escher and P. Neale to establish this method and developed the radiotracer LSC method for micropollutant detection in water.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Polymer Fibre Images
Figure S1: Polymer Fibre Images: (left image) Planar view of PA fibre at 100X magnification; (middle image) Top view of PA fibre at 200X magnification; (right image) schematic sketch of the fibre showing the glass core and the approximate thickness of the polymer coating.
Supplementary information
Supplementary Information
Supplementary Figure 1 (PDF 263 kb)
Rights and permissions
About this article
Cite this article
Bridle, H., Heringa, M. & Schäfer, A. Solid-phase microextraction to determine micropollutant–macromolecule partition coefficients. Nat Protoc 11, 1328–1344 (2016). https://doi.org/10.1038/nprot.2016.068
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.068
This article is cited by
-
Photocatalytic degradation of steroid hormone micropollutants by TiO2-coated polyethersulfone membranes in a continuous flow-through process
Nature Nanotechnology (2022)
-
Separation and degradation detection of nanogram-per-litre concentrations of radiolabelled steroid hormones using combined liquid chromatography and flow scintillation analysis
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.