Abstract
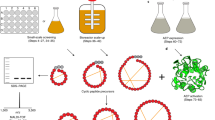
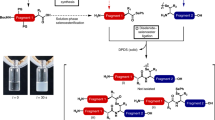
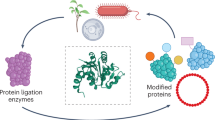
Enzymes that catalyze efficient macrocyclization or site-specific ligation of peptides and proteins can enable tools for drug design and protein engineering. Here we describe a protocol to use butelase 1, a recently discovered peptide ligase, for high-efficiency cyclization and ligation of peptides and proteins ranging in size from 10 to >200 residues. Butelase 1 is the fastest known ligase and is found in pods of the common medicinal plant Clitoria ternatea (also known as butterfly pea). It has a very simple C-terminal-specific recognition motif that requires Asn/Asp (Asx) at the P1 position and a dipeptide His–Val at the P1′ and P2′ positions. Substrates for butelase-mediated ligation can be prepared by standard Fmoc (9-fluorenylmethyloxycarbonyl) chemistry or recombinant expression with the minimal addition of this tripeptide Asn–His–Val motif at the C terminus. Butelase 1 achieves cyclizations that are 20,000 times faster than those of sortase A, a commonly used enzyme for backbone cyclization. Unlike sortase A, butelase is traceless, and it can be used for the total synthesis of naturally occurring peptides and proteins. Furthermore, butelase 1 is also useful for intermolecular ligations and synthesis of peptide or protein thioesters, which are versatile activated intermediates necessary for and compatible with many chemical ligation methods. The protocol describes steps for isolation and purification of butelase 1 from plant extract using a four-step chromatography procedure, which takes ∼3 d. We then describe steps for intramolecular cyclization, intermolecular ligation and butelase-mediated synthesis of protein thioesters. Butelase reactions are generally completed within minutes and often achieve excellent yields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mao, H., Hart, S.A., Schink, A. & Pollok, B.A. Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc. 126, 2670–2671 (2004).
Chang, T.K., Jackson, D.Y., Burnier, J.P. & Wells, J.A. Subtiligase: a tool for semisynthesis of proteins. Proc. Natl. Acad. Sci. USA 91, 12544–12548 (1994).
Wood, D.W. & Camarero, J.A. Intein applications: from protein purification and labeling to metabolic control methods. J. Biol. Chem. 289, 14512–14519 (2014).
Rashidian, M., Dozier, J.K. & Distefano, M.D. Enzymatic labeling of proteins: techniques and approaches. Bioconjug. Chem. 24, 1277–1294 (2013).
Barber, C.J. et al. The two-step biosynthesis of cyclic peptides from linear precursors in a member of the plant family Caryophyllaceae involves cyclization by a serine protease-like enzyme. J. Biol. Chem. 288, 12500–12510 (2013).
Lee, J., McIntosh, J., Hathaway, B.J. & Schmidt, E.W. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J. Am. Chem. Soc. 131, 2122–2124 (2009).
Luo, H. et al. Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in α-amanitin biosynthesis. Chem. Biol. 21, 1610–1617 (2014).
Mazmanian, S.K., Liu, G., Ton-That, H. & Schneewind, O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760–763 (1999).
Nguyen, G.K. et al. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 10, 732–738 (2014).
Serra, A. et al. A high-throughput peptidomic strategy to decipher the molecular diversity of cyclic cysteine-rich peptides. Sci. Rep. 6, 23005 (2016).
Craik, D.J., Daly, N.L., Bond, T. & Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 294, 1327–1336 (1999).
Nguyen, G.K. et al. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J. Biol. Chem. 286, 24275–24287 (2011).
Nguyen, G.K., Lim, W.H., Nguyen, P.Q. & Tam, J.P. Novel cyclotides and uncyclotides with highly shortened precursors from Chassalia chartacea and effects of methionine oxidation on bioactivities. J. Biol. Chem. 287, 17598–17607 (2012).
Nguyen, K.N. et al. Immunostimulating and Gram-negative-specific antibacterial cyclotides from the butterfly pea Clitoria ternatea. FEBS J. 283, 2067–2090 (2016).
Gran, L. On the effect of a polypeptide isolated from 'Kalata-Kalata' (Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol. Toxicol. 33, 400–408 (1973).
Saether, O. et al. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry 34, 4147–4158 (1995).
Tam, J.P. & Lu, Y.A. Synthesis of large cyclic cystine-knot peptide by orthogonal coupling strategy using unprotected peptide precursor. Tetrahedron Lett. 38, 5599–5602 (1997).
Nguyen, G.K. et al. Butelase 1: a versatile ligase for peptide and protein macrocyclization. J. Am. Chem. Soc. 137, 15398–15401 (2015).
Cao, Y., Nguyen, G.K., Tam, J.P. & Liu, C.F. Butelase-mediated synthesis of protein thioesters and its application for tandem chemoenzymatic ligation. Chem. Commun. 51, 17289–17292 (2015).
Nguyen, G.K., Cao, Y., Wang, W., Liu, C.F. & Tam, J.P. Site-specific N-terminal labeling of peptides and proteins using butelase 1 and thiodepsipeptide. Angew. Chem. Int. Ed. Engl. 54, 15694–15698 (2015).
Harris, K.S. et al. Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase. Nat. Commun. 6, 10199 (2015).
Bolscher, J.G. et al. Sortase A as a tool for high-yield histatin cyclization. FASEB J. 25, 2650–2658 (2011).
Dawson, P.E., Muir, T.W., Clark-Lewis, I. & Kent, S.B. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Liu, C.-F. & Tam, J.P. Peptide segment ligation strategy without use of protecting groups. Proc. Natl. Acad. Sci. USA 91, 6584–6588 (1994).
Kolb, H.C., Finn, M.G. & Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Tam, J.P., Lu, Y.A., Liu, C.F. & Shao, J. Peptide synthesis using unprotected peptides through orthogonal coupling methods. Proc. Natl. Acad. Sci. USA 92, 12485–12489 (1995).
Wong, C.T. et al. Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew. Chem. Int. Ed. Engl. 51, 5620–5624 (2012).
Clark, R.J., Akcan, M., Kaas, Q., Daly, N.L. & Craik, D.J. Cyclization of conotoxins to improve their biopharmaceutical properties. Toxicon 59, 446–455 (2012).
Driggers, E.M., Hale, S.P., Lee, J. & Terrett, N.K. The exploration of macrocycles for drug discovery--an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Tam, J.P. & Wong, C.T.T. Chemical synthesis of circular proteins. J. Biol. Chem. 287, 27020–27025 (2012).
Xu, M.Q. & Evans, T.C. Intein-mediated ligation and cyclization of expressed proteins. Methods 24, 257–277 (2001).
Muir, T.W., Sondhi, D. & Cole, P.A. Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. USA 95, 6705–6710 (1998).
Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 10, 411–421 (1999).
Wellings, D.A. & Atherton, E. Methods in Enzymology Vol. 289, 44–67 (Academic Press, 1997).
Acknowledgements
This work was supported by NTU iFood research grant M4081467.080 and Singapore National Research Foundation grant NRFCRP8-2011-05 to J.P.T.
Author information
Authors and Affiliations
Contributions
G.K.T.N. drafted the manuscript. Y.Q. and X.H. developed the protocol for intermolecular ligation. Y.C. and C.-F.L. developed the protocol for butelase-mediated protein thioester synthesis. J.P.T. conceived the idea, supervised the project and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Chromatogram of the C. ternatea extract by preparative anion exchange.
Fractions containing ligase activity are indicated by the double arrow. The right axis indicate the percentage of the elution buffer. The cyan line indicates the gradient used for HPLC separation.
Supplementary Figure 2 A typical MS-guided screening of ligase-containing fractions.
The linear precursor of SFTI has a m/z value of 1766.9 which is converted to 1512.7 in the presence of butelase 1.
Supplementary Figure 3 Chromatogram of the C. ternatea extract by size exclusion chromatography.
Fractions containing ligase activity are indicated by the double arrow.
Supplementary Figure 4 Chromatogram of butelase 1 separation by analytical anion exchange.
Fractions containing ligase activity are indicated by the double arrow. The cyan line indicates the gradient used for HPLC separation.
Supplementary Figure 5 SDS-PAGE analysis of purified butelase 1.
The gel was stained with Coomassie blue. Purified butelase 1 should migrate as a single band with a molecular weight of about 37 kDa.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 673 kb)
Rights and permissions
About this article
Cite this article
Nguyen, G., Qiu, Y., Cao, Y. et al. Butelase-mediated cyclization and ligation of peptides and proteins. Nat Protoc 11, 1977–1988 (2016). https://doi.org/10.1038/nprot.2016.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.118
This article is cited by
-
Nature-inspired protein ligation and its applications
Nature Reviews Chemistry (2023)
-
Butelase-1 as the Prototypical Peptide Asparaginyl Ligase and Its Applications: A Review
International Journal of Peptide Research and Therapeutics (2022)
-
A bifunctional asparaginyl endopeptidase efficiently catalyzes both cleavage and cyclization of cyclic trypsin inhibitors
Nature Communications (2020)
-
Progress toward sourcing plants for new bioconjugation tools: a screening evaluation of a model peptide ligase using a synthetic precursor
3 Biotech (2019)
-
Topology: a unique dimension in protein engineering
Science China Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.