Abstract
For phenotypic behavior to be understood in the context of cell lineage and local environment, properties of individual cells must be measured relative to population-wide traits. However, the inability to accurately identify, track and measure thousands of single cells via high-throughput microscopy has impeded dynamic studies of cell populations. We demonstrate unique labeling of cells, driven by the heterogeneous random uptake of fluorescent nanoparticles of different emission colors. By sequentially exposing a cell population to different particles, we generated a large number of unique digital codes, which corresponded to the cell-specific number of nanoparticle-loaded vesicles and were visible within a given fluorescence channel. When three colors are used, the assay can self-generate over 17,000 individual codes identifiable using a typical fluorescence microscope. The color-codes provided immediate visualization of cell identity and allowed us to track human cells with a success rate of 78% across image frames separated by 8 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Axelrod, R., Axelrod, D.E. & Pienta, K.J. Evolution of cooperation among tumor cells. Proc. Natl. Acad. Sci. USA 103, 13474–13479 (2006).
Eliceiri, K.W. et al. Biological imaging software tools. Nat. Methods 9, 697–710 (2012).
Perlman, Z.E. et al. Multidimensional drug profiling by automated microscopy. Science 306, 1194–1198 (2004).
Rajaram, S., Pavie, B., Wu, L.F. & Altschuler, S.J. PhenoRipper: software for rapidly profiling microscopy images. Nat. Methods 9, 635–637 (2012).
Snijder, B. et al. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature 461, 520–523 (2009).
Snijder, B. & Pelkmans, L. Origins of regulated cell-to-cell variability. Nat. Rev. Mol. Cell Biol. 12, 119–125 (2011).
Ho, M.M., Ng, A.V., Lam, S. & Hung, J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 67, 4827–4833 (2007).
Huang, S. Non-genetic heterogeneity of cells in development: more than just noise. Development 136, 3853–3862 (2009).
Bruchez, M. Jr., Moronne, M., Gin, P., Weiss, S. & Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 281, 2013–2016 (1998).
Jaiswal, J.K., Mattoussi, H., Mauro, J.M. & Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21, 47–51 (2003).
Brown, M.R. et al. Long-term time series analysis of quantum dot encoded cells by deconvolution of the autofluorescence signal. Cytometry A 77, 925–932 (2010).
Parak, W.J., Pellegrino, T. & Plank, C. Labelling of cells with quantum dots. Nanotechnology 16, R9–R25 (2005).
Koo, O.M., Rubinstein, I. & Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine 1, 193–212 (2005).
Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005).
Chan, W.C.W. et al. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 13, 40–46 (2002).
Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 22, 47–52 (2004).
Fournier-Bidoz, S. et al. Facile and rapid one-step mass preparation of quantum-dot barcodes. Angew. Chem. Int. Ed. Engl. 47, 5577–5581 (2008).
Stsiapura, V. et al. Functionalized nanocrystal-tagged fluorescent polymer beads: synthesis, physicochemical characterization, and immunolabeling application. Anal. Biochem. 334, 257–265 (2004).
Han, M., Gao, X., Su, J.Z. & Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 19, 631–635 (2001).
Summers, H.D. et al. Statistical analysis of nanoparticle dosing in a dynamic cellular system. Nat. Nanotechnol. 6, 170–174 (2011).
Scita, G. & Di Fiore, P.P. The endocytic matrix. Nature 463, 464–473 (2010).
Errington, R.J. et al. Single cell nanoparticle tracking to model cell cycle dynamics and compartmental inheritance. Cell Cycle 9, 121–130 (2010).
Wilhelm, C., Gazeau, F., Roger, J., Pons, J.N. & Bacri, J.-C. Interaction of anionic superparamagnetic nanoparticles with cells: kinetic analyses of membrane adsorption and subsequent internalization. Langmuir 18, 8148–8155 (2002).
Tonkin, J.A. et al. Automated cell identification and tracking using nanoparticle moving-light-displays. PLoS ONE 7, e40835 (2012).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, 695–702 (2008).
Carpenter, A.E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Kim, J.A., Åberg, C., Salvati, A. & Dawson, K.A. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 7, 62–68 (2012).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council, UK, under grant EP/H008683/1, 'Nanoparticle Cytometrics'. P.R. was supported by the Engineering and Physical Sciences Research Council, UK International Collaboration Sabbatical scheme under grant EP/J00619X/1 and the European Regional Development Funded, Swansea Centre for Nanohealth (CNH).
Author information
Authors and Affiliations
Contributions
P.R., H.D.S., A.E.C. and M.R.B. designed the experiments; P.R., M.R.B., J.T. and V.M. analyzed the data; and P.R., A.E.C. and H.D.S. wrote the manuscript in close collaboration with the other coauthors. M.D.H. implemented the cell culture dosing regimen; J.W.W., M.R.B. and V.M. performed the microscopy measurements and the image analysis. N.H., A.B. and R.B. carried out the TEM and EDX studies. All coauthors discussed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
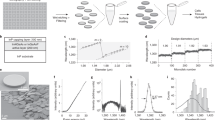
Supplementary Figure 1 Distributions obtained after loading U-2 OS cells for 15 minutes and 3 h with 705-nm quantum dots.
(a) Frequency distribution of 705 nm nanoparticle labelled endosomes after 15 minutes exposure at 10 nM concentration. (b) Frequency distribution of nanoparticle labelled endosomes after 3 hour exposure at 10 nM concentration. The broken dotted line shows the best fit to a Poisson (random) uptake model and the solid line represents the fit to a negative binomial distribution which demonstrate the over dispersion in the process. The similarity of the r and p values for the negative binomial fits between 15 minute and 3 hour exposure indicates that uptake is very quick and the majority of quantum dots have been internalized after 15 minutes.
Supplementary Figure 2 TEM EDX analysis to prove each endosome or lysosome contains only one color of QD.
The quantum dot exposed U-2 OS cells (each color quantum dot is exposed to cells in culture media for 15 min, with a 15 min incubate between colors and a final 3 hr incubate) were harvested and placed in fixative (2% glutaraldehyde and 2% formaldehyde in 100 mM PIPES buffer), washed in a buffer, then spun into pellets, and fixed in 2% osmium tetroxide. Following dehydration by a series of ascending strength alcohols and washing with dry acetone, the specimens were infiltrated with Spurr’s resin which was polymerized at 60 °C for 24 h. Sections were cut from the polymerized block with a nominal thickness of 100 nm using an ultramicrotome (Leica Ultracut E) and placed on a copper grid (Agar Scientific). No conventional heavy metal stain (uranyl acetate or lead citrate) was used. Transmission electron microscopy (TEM) was conducted on an FEI Tecnai F20 field emission gun (FEG)-TEM operating at 200 kV and fitted with a Gatan Orius SC600A CCD camera and an Oxford Instruments energy dispersive X-ray (EDX) detector with standardless elemental quantification in the INCA software package (Oxford Instruments). (a) Whole cell TEM image together with the magnified region showing three vesicles loaded with different quantum dots. Successive magnification of the 525 nm (b), 705 nm (c) and 585 nm (d) quantum dot loaded vesicles together with the EDX spectrum confirming the chemical composition.
Supplementary Figure 3 Uptake histograms of 705-nm nanoparticles for U-2 OS, A549, AHH-1 and BEAS-2B cells.
The endocytic capacity of cells will limit the number of quantum dots a cell can take up and therefore limit the number of individual codes that can be generated. We have studied the uptake of nanoparticles by several different cell lines, for example we have measured the uptake of nanoparticles (5 nM dose) on 3 fundamentally different cell types; two adherent cancer cell lines – U-2 OS osteosarcoma (bone cancer) (a) and A549 epithelial lung cancer (b), AHH-1 lympoblastoid - a suspension cell line (c) and BEAS-2B epithelial lung non-cancerous (d). All of these very different cell lines demonstrate nanoparticle uptake even if the amounts and distributions vary slightly. These should be compared with the 5 nM dose histograms (Supplementary Fig. 5) – peak NLV values of 5 - 8 and the distributions are similar in spread.
Supplementary Figure 4 Cell population barcoding for microscopy.
Nanoparticle vesicle encoding for labeling heterogeneous subpopulations using U-2 OS cells. (a) A representation of a 16X20 array of microscopy fields of view, where each point represents one cell and the color denotes the RGB representation of the three color NLV code. The circle represents the average distance from a cell a duplicate cell would appear (if a duplicate existed). (b) A dot plot of the total number of NLVs per cell versus the cell’s nuclear area shows again the color of each point as the RGB representation of NLV content of the cell. The U-2 OS cell line contains polyploid cells; these are cells that go through all the phases of the cell cycle but rather than dividing at mitosis continues as a single cell (with double the normal DNA content etc.). As a result these cells are abnormally large. We found that cells with smaller nuclei have fewer NLVs. However there is a subpopulation of cells which have a distinct light blue color on the dot plot due to high values in the RGB code, particularly the 525 nm NLV. This is to be expected as it has been shown for certain cell lines that uptake of nanoparticles is proportional to the cell area24. (c) One field of view is shown, highlighting a polyploid cell with the white arrow which is easily recognizable from the dot plot or the wide field representation of the cell population due to its light blue coloration. The same cell is highlighted by black arrows in (a) and (b). The correlation of the high loading of nanoparticles to polyploidy can be confirmed by the large nuclear area of the cells. Therefore the cell RGB color is indicative of a cells’ ploidal-state and gives a straightforward visualization of the phenotype and its relation to the overall heterogeneity of the cell population.
Supplementary Figure 5 Statistical analysis of different color loading of U-2 OS cells for a 5 nM dose.
A repeat of the statistical analysis of the 10 nM dose loading experiment (Fig. 2a–g) but for a reduced 5 nM dose for the 3 quantum dot colors (a-c) Histograms display the number of cells loaded with 585 nm, 705 nm and 525 nm NLVs, respectively for a reduced 5 nM dose. The solid line represents fits to a real valued negative binomial with parameters defined by r and p (Online Methods). The negative binomial fit highlights over dispersion in the NLV per cell and the similar parameters obtained indicate similar loadings for each color. (d-f) Correlation plots between NLV numbers are shown for each pair of the three different NLV colors, for the total cell population together with the Pearson correlation value (g) Histogram of the percentage of cells which share the same code with other cells in the population. In three separate dosing experiments, 10% have unique codes (red) and the remainder shares a code with a small number of cells in the experiment, error bars representing the standard deviation are included to demonstrate repeatability.
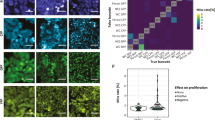
Supplementary Figure 6 Temporal and generational tracking of U-2 OS cells with no membrane stain over many-hour intervals by microscopy.
We tested the nanoparticle vesicle encoding approach to track U-2 OS cells within a 5X5 field of view measured using the InCell high-throughput microscope over the course of 5 hours in culture (Supplementary Movie). (a) Cells were imaged (5X5 frames) and the NLV-based bar codes measured at time 0 after nanoparticle washout. At intervals increasing from 15 minutes to 4.5 hours we attempted to match every cell to a cell labeled in frame 1 based purely on its bar code and not on tracking data from intervening time points; the accuracy of this assignment (blue) is ~80% after 4.5 hours. For comparison we compare our tracking method with the cell tracking algorithms available in CellProfiler. The most successful algorithm (see Supplementary Note 7) of those available most successful algorithms was the ‘distance’ nearest neighbor method (red). Clearly counting the exact number NLV in live cells using fluorescent microscopy is difficult due to changes in focus, cell shape etc. (as for the Confocal Microscope experiment with A549 cells we observe a fluctuation in NLV frame to frame with a mean of 0 and a standard deviation of 1.5 NLV) and also there is the possibility that NLV could merge over the long term or be ejected from the cell, however the number of unique codes generated means simply matching the closest code gives robust tracking. Although for this reason we ignored cells which have low NLV numbers (NLV<3, approximately 15% of cells observed in this case). (b) and (c) Examples of images used to identify the original progenitor cells in the image at 0 hour from the daughter cells at time 4.5 hours. The number of colored NLVs in the cells are also given. Daughter cells produced by the two mitotic events can be linked to their parent cells through a simple algorithm which bases the choice of parent-daughter on minimization of the summed difference between labelled endosomes in all three color channels. Implementation of this approach across a number of image frames correctly identified daughter-parent relationships in 14 out of 16 division events as compared with the ground truth obtained by manually observing mitosis events in the brightfield images. The time step of 4.5 hours is a relatively small fraction of the cell cycle and so there are only a few mitotic events within each frame. Nevertheless the barcoding technique still demonstrates its utility as a powerful tool with which to identify inter-generational relationships; correctly assigning daughters to parent in 87% of cases, without any knowledge (i.e. spatial or temporal) of the division processes which create them. For tracking over several generations we note that as cells divide, the number of colored vesicles are diluted in each cell and therefore the codes become less and less unique with each mitosis event, however applying a further dose of quantum dots could solve this problem.
Supplementary Figure 7 Typical confocal microscope image of cells tracked using the color-coded NLVs.
A typical confocal microscope image of cells which we use to validate our tracking method, in this case each field has approximately 80-90 cells.
Supplementary Figure 8 Tracking success for state-of-the-art tracking algorithms.
We have compared our tracking method with the best open source tracking algorithms currently available in the freely available CellProfiler software (http://www.cellprofiler.org/). We use the TrackObjects module which will search within a maximum specified distance (see the distance within which to search setting) of the object's location in the previous image, looking for a "match". Objects that match are assigned the same number, or label, throughout the entire movie. Normally the user would have to segment the cells in CellProfiler: however to give the tracking algorithm the maximum chance we have manually segmented each cell and therefore the results ignore the fact segmentation errors significantly reduce the success of these algorithms. CellProfiler employs 3 tracking methods based on image tracking. (i) Overlap: Compares the amount of spatial overlap between identified objects in the previous frame with those in the current frame (red curve). (ii) Distance: Compares the distance between each identified object in the previous frame with that of the current frame (blue curve). The closest objects to each other will be assigned the same number (label). Distances are measured from the perimeter of each object. This method performs the best of the algorithms although becomes increasingly dependent on luck as the time intervals increase, consequently accuracy decreases with larger intervals (as would be expected). (iii) LAP: Uses the linear assignment problem (LAP) framework. The linear assignment problem (LAP) algorithm28 addresses the challenges of high object density, motion heterogeneity, temporary disappearances, and object merging and splitting. The algorithm first links objects between consecutive frames and then links the resulting partial trajectories into complete trajectories. Both steps are formulated as global combinatorial optimization problems whose solution identifies the overall most likely set of object trajectories throughout a movie. Tracks are constructed from an image sequence by detecting objects in each frame and linking objects between consecutive frames as a first step. This step alone may result in incompletely tracked objects due to the appearance and disappearance of objects, either in reality or apparently because of noise and imaging limitations. To correct this, you may apply an optional second step which closes temporal gaps between tracked objects and captures merging and splitting events. This step takes place at the end of the analysis run. Designed to address challenges in high object density, temporary disappearances, non-uniform motion and the merging and splitting of objects. An initial pass links objects between consecutive frames and then, using these links, the partial trajectories are linked together into complete trajectories. This algorithm gives identical results to the ‘distance’ metric in our case as there are no incomplete tracks.
Supplementary Figure 9 Histogram illustrating the average fluctuation of each color NLV measured per cell between 1-h time frames.
A histogram which gives the distribution of the average fluctuation of each color NLV measured per cell between 1 hour time frames averaged over the 8 hour time period. The standard deviation of the NLV fluctuation is slightly below 1.5 NLV per color per cell between 1 hour frames. There is no drift in the NLV number over time (mean = 0) which was confirmed by plotting the histogram at each time interval and comparing.
Supplementary Figure 10 Nanoparticle vesicle encoding for labeling heterogeneous subpopulations using U-2 OS cells with NLV fluctuation included.
A representation of a 16X20 array of microscopy fields of view, where each point represents one cell and the color denotes the RGB representation of the three color NLV code. The solid circle (radius 8 fields-of-view) represents the average distance from a cell a duplicate cell would appear (if a duplicate existed). If however we correct for the fact that each color NLV is allowed to fluctuate by +/-3 (twice the standard deviation in NLV fluctuation (Supplementary Fig. 9) then the dashed line circle (radius 1.4 field-of-view) represents the mean distance before a cell erroneously appears to have the same code. Even though the circle is significantly smaller as it is still covers an area large than a single field of view the tracking method still achieves a high accuracy because we only consider matches between cells in a single field of view.
Supplementary Figure 11 Tracking success rate for comparison over intervals between U-2 OS cells with fewer quantum-dot colors.
Tracking success rate for comparison over intervals between 0.5 and 5 hours using 3 (585 nm, 705 nm and 525 nm) (Supplementary Fig. 6), 2 (705 nm and 525 nm) and 1 color (525 nm) quantum dot.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1852 kb)
Time lapse video for a typical 2 × 2 field of view
The video shows a typical 2 × 2 field of view obtained using the InCell high-throughput microscope. The U-2 OS cells have been loaded with the 3 color quantum dots and the cells are labelled according to the false color barcode is allocated due to the number of colored NLV per cell. (WMV 3009 kb)
Supplementary Software
Algorithm for approximating the possible number of codes using different colored QDs and different correlations between these loadings. Algorithm for counting the NLV per cell and tracking between time frames. (ZIP 4279 kb)
Source data
Rights and permissions
About this article
Cite this article
Rees, P., Wills, J., Brown, M. et al. Nanoparticle vesicle encoding for imaging and tracking cell populations. Nat Methods 11, 1177–1181 (2014). https://doi.org/10.1038/nmeth.3105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3105
This article is cited by
-
Imaging flow cytometry
Nature Reviews Methods Primers (2022)
-
Multiplexed laser particles for spatially resolved single-cell analysis
Light: Science & Applications (2019)
-
The origin of heterogeneous nanoparticle uptake by cells
Nature Communications (2019)
-
Microscopic inspection and tracking of single upconversion nanoparticles in living cells
Light: Science & Applications (2018)
-
Lasing in Live Mitotic and Non-Phagocytic Cells by Efficient Delivery of Microresonators
Scientific Reports (2017)