Abstract
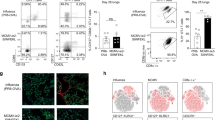
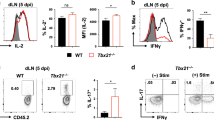
Inflammation induced by recognition of pathogen-associated molecular patterns markedly affects subsequent adaptive responses. We asked whether the adaptive immune system can also affect the character and magnitude of innate inflammatory responses. We found that the response of memory, but not naive, CD4+ T cells enhances production of multiple innate inflammatory cytokines and chemokines (IICs) in the lung and that, during influenza infection, this leads to early control of virus. Memory CD4+ T cell–induced IICs and viral control require cognate antigen recognition and are optimal when memory cells are either T helper type 1 (TH1) or TH17 polarized but are independent of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) production and do not require activation of conserved pathogen recognition pathways. This represents a previously undescribed mechanism by which memory CD4+ T cells induce an early innate response that enhances immune protection against pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Janeway, C.A. Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Pulendran, B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 199, 227–250 (2004).
Pulendran, B., Palucka, K. & Banchereau, J. Sensing pathogens and tuning immune responses. Science 293, 253–256 (2001).
Powell, T.J. et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J. Immunol. 178, 1030–1038 (2007).
Swain, S.L. et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol. Rev. 211, 8–22 (2006).
Rogers, P.R., Dubey, C. & Swain, S.L. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 (2000).
London, C.A., Lodge, M.P. & Abbas, A.K. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 164, 265–272 (2000).
Bradley, L.M., Duncan, D.D., Yoshimoto, K. & Swain, S.L. Memory effectors: a potent, IL-4–secreting helper T cell population that develops in vivo after restimulation with antigen. J. Immunol. 150, 3119–3130 (1993).
Dahl, M.E., Dabbagh, K., Liggitt, D., Kim, S. & Lewis, D.B. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat. Immunol. 5, 337–343 (2004).
Didierlaurent, A. et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 205, 323–329 (2008).
Chace, J.H., Cowdery, J.S. & Field, E.H. Effect of anti-CD4 on CD4 subsets. I. Anti-CD4 preferentially deletes resting, naive CD4 cells and spares activated CD4 cells. J. Immunol. 152, 405–412 (1994).
Scott, B. et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity 1, 73–83 (1994).
McKinstry, K.K. et al. Rapid default transition of CD4 T cell effectors to functional memory cells. J. Exp. Med. 204, 2199–2211 (2007).
Thomas, P.G. et al. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc. Natl. Acad. Sci. USA 103, 2764–2769 (2006).
Mayer, K.D. et al. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-γ production. J. Immunol. 174, 7732–7739 (2005).
Boehm, U., Klamp, T., Groot, M. & Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15, 749–795 (1997).
Farber, J.M. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61, 246–257 (1997).
Amichay, D. et al. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-γ with patterns of tissue expression that suggest nonredundant roles in vivo. J. Immunol. 157, 4511–4520 (1996).
Nakanishi, Y., Lu, B., Gerard, C. & Iwasaki, A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature 462, 510–513 (2009).
Brown, D.M., Dilzer, A.M., Meents, D.L. & Swain, S.L. CD4 T cell–mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177, 2888–2898 (2006).
McKinstry, K.K. et al. IL-10 deficiency unleashes an influenza-specific TH17 response and enhances survival against high-dose challenge. J. Immunol. 182, 7353–7363 (2009).
Wakim, L.M., Waithman, J., van Rooijen, N., Heath, W.R. & Carbone, F.R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Debbabi, H. et al. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L274–L279 (2005).
Lemos, M.P., Fan, L., Lo, D. & Laufer, T.M. CD8α+ and CD11b+ dendritic cell–restricted MHC class II controls TH1 CD4+ T cell immunity. J. Immunol. 171, 5077–5084 (2003).
Diebold, S.S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Imai, Y. et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 (2008).
Pichlmair, A. et al. RIG-I–mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Guarda, G. et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 460, 269–273 (2009).
Kim, K.D. et al. Adaptive immune cells temper initial innate responses. Nat. Med. 13, 1248–1252 (2007).
Salomon, R., Hoffmann, E. & Webster, R.G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. USA 104, 12479–12481 (2007).
Tuvim, M.J., Evans, S.E., Clement, C.G., Dickey, B.F. & Gilbert, B.E. Augmented lung inflammation protects against influenza A pneumonia. PLoS One 4, e4176 (2009).
Kalinski, P. & Moser, M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat. Rev. Immunol. 5, 251–260 (2005).
Hale, B.G., Randall, R.E., Ortin, J. & Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89, 2359–2376 (2008).
Joffre, O., Nolte, M.A., Sporri, R. & Reis e Sousa, C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 (2009).
Dienz, O. et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 206, 69–78 (2009).
Szretter, K.J. et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81, 2736–2744 (2007).
Schmitz, N., Kurrer, M., Bachmann, M.F. & Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79, 6441–6448 (2005).
Lee, S.W., Youn, J.W., Seong, B.L. & Sung, Y.C. IL-6 induces long-term protective immunity against a lethal challenge of influenza virus. Vaccine 17, 490–496 (1999).
Hama, Y. et al. Interleukin 12 is a primary cytokine responding to influenza virus infection in the respiratory tract of mice. Acta Virol. 53, 233–240 (2009).
GeurtsvanKessel, C.H. & Lambrecht, B.N. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 1, 442–450 (2008).
McGill, J., Heusel, J.W. & Legge, K.L. Innate immune control and regulation of influenza virus infections. J. Leukoc. Biol. 86, 803–812 (2009).
Monteiro, J.M., Harvey, C. & Trinchieri, G. Role of interleukin-12 in primary influenza virus infection. J. Virol. 72, 4825–4831 (1998).
Zhao, M.Q. et al. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8+ T cell recognition. J. Clin. Invest. 106, R49–R58 (2000).
Guidotti, L.G. & Chisari, F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19, 65–91 (2001).
Le Saout, C., Mennechet, S., Taylor, N. & Hernandez, J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc. Natl. Acad. Sci. USA 105, 19414–19419 (2008).
Elyaman, W. et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am. J. Pathol. 173, 411–422 (2008).
Latham, K.A., Whittington, K.B., Zhou, R., Qian, Z. & Rosloniec, E.F. Ex vivo characterization of the autoimmune T cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic joints. J. Immunol. 174, 3978–3985 (2005).
Strutt, T.M., Uzonna, J., McKinstry, K.K. & Bretscher, P.A. Activation of thymic T cells by MHC alloantigen requires syngeneic, activated CD4+ T cells and B cells as APC. Int. Immunol. 18, 719–728 (2006).
Acknowledgements
This work was supported by the US National Institutes of Health (P01AI04630 to and P01AI04566 to S.L.S.), the US Department of Defense (HR#3222) and the Trudeau Institute. We thank J. Kohlmeier and D. Woodland (Trudeau Institute) for Ccr5−/− and Ifnar2−/− mice and M. Mohrs (Trudeau Institute) for C57BL/6-Tg (IFN-γ–EYFP) mice. Influenza A/Philippines was obtained from S. Epstein (CBER FDA), and engineered virus A/PR8-OVAII was obtained from P. Doherty (University of Melbourne). LPS-free whole OVA protein was a generous gift from T. Moran (Mount Sinai School of Medicine).
Author information
Authors and Affiliations
Contributions
T.M.S. and K.K.M. contributed equally to the design, processing, collection and analysis of data and, together with S.L.S., wrote the paper. S.L.S. and R.W.D. contributed to study design. J.P.D., C.W., Y.K., J.D.C. and G.H. processed and collected data. All authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 863 kb)
Rights and permissions
About this article
Cite this article
Strutt, T., McKinstry, K., Dibble, J. et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med 16, 558–564 (2010). https://doi.org/10.1038/nm.2142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2142
This article is cited by
-
High-dimensional analysis reveals an immune atlas and novel neutrophil clusters in the lungs of model animals with Actinobacillus pleuropneumoniae-induced pneumonia
Veterinary Research (2023)
-
Role of the Hematopoietic Stem Cells in Immunological Memory
Current Stem Cell Reports (2022)
-
T-bet optimizes CD4 T-cell responses against influenza through CXCR3-dependent lung trafficking but not functional programming
Mucosal Immunology (2019)
-
Ergoferon Increases IL-2 Production by Activated Lymphocytes
Bulletin of Experimental Biology and Medicine (2019)
-
IL-15 supports the generation of protective lung-resident memory CD4 T cells
Mucosal Immunology (2018)