Abstract
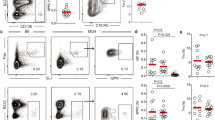
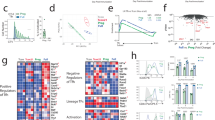
B lymphocytes differentiate into antibody-secreting cells under the antigen-specific control of follicular helper T cells (TFH cells). Here we demonstrate that isotype-switched plasma cells expressed major histocompatibility complex (MHC) class II, the costimulatory molecules CD80 and CD86, and the intracellular machinery required for antigen presentation. Antigen-specific plasma cells accessed, processed and presented sufficient antigen in vivo to induce multiple helper T cell functions. Notably, antigen-primed plasma cells failed to induce interleukin 21 (IL-21) or the transcriptional repressor Bcl-6 in naive helper T cells and actively decreased these key molecules in antigen-activated TFH cells. Mice lacking plasma cells showed altered TFH cell activity, which provided evidence of this negative feedback loop. Hence, antigen presentation by plasma cells defines a previously unknown layer of cognate regulation that limits the antigen-specific TFH cell program that controls ongoing B cell immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
MacLennan, I.C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 (2005).
Fazilleau, N., Mark, L., McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Follicular helper T cells: lineage and location. Immunity 30, 324–335 (2009).
King, C., Tangye, S.G. & Mackay, C.R. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26, 741–766 (2008).
Vinuesa, C.G., Sanz, I. & Cook, M.C. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 9, 845–857 (2009).
Ansel, K.M., McHeyzer-Williams, L.J., Ngo, V.N., McHeyzer-Williams, M.G. & Cyster, J.G. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 8, 753–761 (2007).
Fazilleau, N., McHeyzer-Williams, L.J., Rosen, H. & McHeyzer-Williams, M.G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10, 375–384 (2009).
Vinuesa, C.G., Tangye, S.G., Moser, B. & Mackay, C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5, 853–865 (2005).
Linterman, M.A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Poholek, A.C. et al. In vivo regulation of Bcl6 and T follicular helper cell development. J. Immunol. 185, 313–326 (2010).
Good-Jacobson, K.L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11, 535–542 (2010).
McHeyzer-Williams, L.J., Cool, M. & McHeyzer-Williams, M.G. Antigen-specific B cell memory: expression and replenishment of a novel B220− memory B cell compartment. J. Exp. Med. 191, 1149–1166 (2000).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998).
Qi, H., Cannons, J.L., Klauschen, F., Schwartzberg, P.L. & Germain, R.N. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, 1047–1061 (2005).
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002).
Spolski, R. & Leonard, W.J. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr. Opin. Immunol. 20, 295–301 (2008).
Jacob, J. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 176, 679–687 (1992).
McHeyzer-Williams, M.G., McLean, M.J., Lalor, P.A. & Nossal, G.J. Antigen-driven B cell differentiation in vivo. J. Exp. Med. 178, 295–307 (1993).
Ho, F., Lortan, J.E., MacLennan, I.C. & Khan, M. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16, 1297–1301 (1986).
Slifka, M.K., Antia, R., Whitmire, J.K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Shapiro-Shelef, M. et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620 (2003).
Martins, G. & Calame, K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu. Rev. Immunol. 26, 133–169 (2008).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Todd, D.J. et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206, 2151–2159 (2009).
Ozaki, K. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173, 5361–5371 (2004).
Kwon, H. et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 31, 941–952 (2009).
Piskurich, J.F. et al. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat. Immunol. 1, 526–532 (2000).
McHeyzer-Williams, M.G., Nossal, G.J. & Lalor, P.A. Molecular characterization of single memory B cells. Nature 350, 502–505 (1991).
Dadaglio, G., Nelson, C.A., Deck, M.B., Petzold, S.J. & Unanue, E.R. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity 6, 727–738 (1997).
Denzin, L.K., Fallas, J.L., Prendes, M. & Yi, W. Right place, right time, right peptide: DO keeps DM focused. Immunol. Rev. 207, 279–292 (2005).
Trombetta, E.S. & Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23, 975–1028 (2005).
Ellyard, J.I. et al. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood 103, 3805–3812 (2004).
Gonzalez-Garcia, I., Ocana, E., Jimenez-Gomez, G., Campos-Caro, A. & Brieva, J.A. Immunization-induced perturbation of human blood plasma cell pool: progressive maturation, IL-6 responsiveness, and high PRDI-BF1/BLIMP1 expression are critical distinctions between antigen-specific and nonspecific plasma cells. J. Immunol. 176, 4042–4050 (2006).
Yi, Q., Dabadghao, S., Osterborg, A., Bergenbrant, S. & Holm, G. Myeloma bone marrow plasma cells: evidence for their capacity as antigen-presenting cells. Blood 90, 1960–1967 (1997).
Crotty, S., Johnston, R.J. & Schoenberger, S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11, 114–120 (2010).
Allen, C.D., Okada, T., Tang, H.L. & Cyster, J.G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Reinhardt, R.L., Liang, H.E. & Locksley, R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
Mohr, E. et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 182, 2113–2123 (2009).
Angelin-Duclos, C., Cattoretti, G., Lin, K.I. & Calame, K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 165, 5462–5471 (2000).
Gatto, D., Paus, D., Basten, A., Mackay, C.R. & Brink, R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31, 259–269 (2009).
Yusuf, I. et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol. 185, 190–202 (2010).
Acknowledgements
We thank L. Denzin (Memorial Sloan Kettering Cancer Center) for antibodies 2C3A and Ob1; A. Rudensky (Memorial Sloan Kettering Cancer Center) for monoclonal antibody 15G4; E. Unanue (Washington University) for the Aw3.18 cell line; K. Calame (Columbia University) for mice with loxP-flanked Prdm1 alleles excised by Cre recombinase expressed from a CD19 promoter; and K. Spencer for help with confocal imaging. Supported by the German Academic Exchange Service (E.U.), the German Research Foundation (E.U.), the Canadian Institutes of Health Research (MFE-98574 to K.A.W.) and the US National Institutes of Health (AI047231, AI040215 and AI071182 to M.G.M.-W.). This is The Scripps Research Institute manuscript 20113.
Author information
Authors and Affiliations
Contributions
N.P., L.J.M.-W. and M.G.M.-W. conceived of and designed the project; N.P. provided and analyzed the data for all experiments except Figures 3a,b and 6; K.A.W. designed and completed the experiments and analyzed the data for pMHCII HEL-specific plasma cells (Fig. 3a,b); E.U. laid the experimental foundation for staining HEL-specific B cells; N.F. prepared the activated helper T cell samples for quantitative PCR analysis (Fig. 4b) and provided expertise in setting up the T cell in vitro experiments; L.J.M.-W. and M.G.M.-W. did the experiments for and analyzed the data from the Blimp-1-cKO mice (Fig. 6); K.A.W. and N.F. contributed ideas and participated in the manuscript preparation; and N.P., L.J.M.-W. and M.G.M.-W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Table 1 (PDF 16592 kb)
Rights and permissions
About this article
Cite this article
Pelletier, N., McHeyzer-Williams, L., Wong, K. et al. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol 11, 1110–1118 (2010). https://doi.org/10.1038/ni.1954
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1954
This article is cited by
-
Increased plasmablasts enhance T cell-mediated beta cell destruction and promote the development of type 1 diabetes
Molecular Medicine (2022)
-
Differential immune cell infiltrations between healthy periodontal and chronic periodontitis tissues
BMC Oral Health (2020)
-
Infection-induced plasmablasts are a nutrient sink that impairs humoral immunity to malaria
Nature Immunology (2020)
-
Function and dysfunction of plasma cells in intestine
Cell & Bioscience (2019)
-
Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis
Stem Cell Research & Therapy (2019)