Abstract
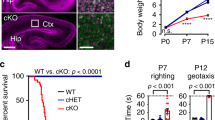
Topoisomerase II (TOP2) removes torsional stress from DNA and facilitates gene transcription by introducing transient DNA double-strand breaks (DSBs). Such DSBs are normally rejoined by TOP2 but on occasion can become abortive and remain unsealed. Here we identify homozygous mutations in the TDP2 gene encoding tyrosyl DNA phosphodiesterase-2, an enzyme that repairs 'abortive' TOP2-induced DSBs, in individuals with intellectual disability, seizures and ataxia. We show that cells from affected individuals are hypersensitive to TOP2-induced DSBs and that loss of TDP2 inhibits TOP2-dependent gene transcription in cultured human cells and in mouse post-mitotic neurons following abortive TOP2 activity. Notably, TDP2 is also required for normal levels of many gene transcripts in developing mouse brain, including numerous gene transcripts associated with neurological function and/or disease, and for normal interneuron density in mouse cerebellum. Collectively, these data implicate chromosome breakage by TOP2 as an endogenous threat to gene transcription and to normal neuronal development and maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Cortes Ledesma, F., El-Khamisy, S.F., Zuma, M.C., Osborn, K. & Caldecott, K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461, 674–678 (2009).
Calabrese, R., Capriotti, E., Fariselli, P., Martelli, P.L. & Casadio, R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum. Mutat. 30, 1237–1244 (2009).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Rodrigues-Lima, F., Josephs, M., Katan, M. & Cassinat, B. Sequence analysis identifies TTRAP, a protein that associates with CD40 and TNF receptor–associated factors, as a member of a superfamily of divalent cation-dependent phosphodiesterases. Biochem. Biophys. Res. Commun. 285, 1274–1279 (2001).
Pype, S. et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor–associated factors (TRAFs), and that inhibits nuclear factor-κB activation. J. Biol. Chem. 275, 18586–18593 (2000).
Gómez-Herreros, F. et al. TDP2-dependent non-homologous end-joining protects against topoisomerase II–induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 9, e1003226 (2013).
Zeng, Z. et al. TDP2 promotes repair of topoisomerase I–mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 40, 8371–8380 (2012).
Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 9, 327–337 (2009).
Vos, S.M., Tretter, E.M., Schmidt, B.H. & Berger, J.M. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 12, 827–841 (2011).
Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350 (2009).
Pommier, Y., Leo, E., Zhang, H. & Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433 (2010).
Schellenberg, M.J. et al. Mechanism of repair of 5′-topoisomerase II–DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nat. Struct. Mol. Biol. 19, 1363–1371 (2012).
Shi, K. et al. Structural basis for recognition of 5′-phosphotyrosine adducts by Tdp2. Nat. Struct. Mol. Biol. 19, 1372–1377 (2012).
Esguerra, C.V. et al. Ttrap is an essential modulator of Smad3-dependent Nodal signaling during zebrafish gastrulation and left-right axis determination. Development 134, 4381–4393 (2007).
Takashima, H. et al. Mutation of TDP1, encoding a topoisomerase I–dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 32, 267–272 (2002).
Katyal, S. et al. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 26, 4720–4731 (2007).
Hirano, R. et al. Spinocerebellar ataxia with axonal neuropathy: consequence of a Tdp1 recessive neomorphic mutation? EMBO J. 26, 4732–4743 (2007).
Lee, Y. et al. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nat. Neurosci. 12, 973–980 (2009).
King, I.F. et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature 501, 58–62 (2013).
Lyu, Y.L. et al. Role of topoisomerase IIβ in the expression of developmentally regulated genes. Mol. Cell. Biol. 26, 7929–7941 (2006).
Ju, B.-G. et al. A topoisomerase IIβ–mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006).
Thakurela, S. et al. Gene regulation and priming by topoisomerase IIα in embryonic stem cells. Nat. Commun. 4, 2478 (2013).
Tiwari, V.K. et al. Target genes of topoisomerase IIβ regulate neuronal survival and are defined by their chromatin state. Proc. Natl. Acad. Sci. USA 109, E934–E943 (2012).
Haffner, M.C. et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675 (2010).
LoTurco, J.J., Owens, D.F., Heath, M.J., Davis, M.B. & Kriegstein, A.R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298 (1995).
Liu, X., Wang, Q., Haydar, T.F. & Bordey, A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8, 1179–1187 (2005).
Edgar, R., Domrachev, M. & Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Pelak, K. et al. The characterization of twenty sequenced human genomes. PLoS Genet. 6, e1001111 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Ge, D. et al. SVA: software for annotating and visualizing sequenced human genomes. Bioinformatics 27, 1998–2000 (2011).
Robinson, J.T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Klambauer, G. et al. cn.MOPS: mixture of Poissons for discovering copy number variations in next-generation sequencing data with a low false discovery rate. Nucleic Acids Res. 40, e69 (2012).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 (2000).
de Brouwer, A.P.M., van Bokhoven, H. & Kremer, H. Comparison of 12 reference genes for normalization of gene expression levels in Epstein-Barr virus–transformed lymphoblastoid cell lines and fibroblasts. Mol. Diagn. Ther. 10, 197–204 (2006).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC T) method. Methods 25, 402–408 (2001).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Wall, F.E., Henkel, R.D., Stern, M.P., Jenson, H.B. & Moyer, M.P. An efficient method for routine Epstein-Barr virus immortalization of human B lymphocytes. In Vitro Cell. Dev. Biol. Anim. 31, 156–159 (1995).
Lee, H.-Y., Greene, L.A., Mason, C.A. & Manzini, M.C. Isolation and culture of post-natal mouse cerebellar granule neuron progenitor cells and neurons. J. Vis. Exp. 23, e990 (2009).
Thomson, G. et al. Generation of assays and antibodies to facilitate the study of human 5′-tyrosyl DNA phosphodiesterase. Anal. Biochem. 436, 145–150 (2013).
Acknowledgements
We thank the patients and their family for their cooperation in this research project, N. Sabry for the provision of blood from the Egyptian patient, J.H.J. Hoeijmakers for critical evaluation of the manuscript, T. van Moorsel and L. Ju for technical assistance and D. Huylebroeck for the provision of unpublished behavioral data on Tdp2Δ1–3 mice. Microarray analysis was conducted by M. Hubank and colleagues at University College London (UCL) Genomics, UCL Institute of Child Health. We also thank D.B. Goldstein, E.L. Heinzen and the Duke Center for Human Genome Variation Genetic Analysis Facility, and we thank the following individuals associated with the Carol Woods and Crosdaile Retirement Communities, the MURDOCK Study Community Registry and Biorepository, and the Washington University Neuromuscular Genetics Project: J. McEvoy, A. Need, J. Silver, M. Silver; E.T. Cirulli, V. Dixon, D.K. Attix, O. Chiba-Falek, K. Schmader, S. McDonald, H.K. White, M. Yanamadala, C. Depondt, S. Sisodiya, W.B. Gallentine, A.M. Husain, M.A. Mikati, R.A. Radtke, S.R. Sinha, J. Hoover-Fong, N.L. Sobreira, D. Valle, D. Daskalakis, W.L. Lowe, V. Shashi, K. Schoch, D.H. Murdock, S.M. Palmer, Z. Farfel, D.D. Lancet, E. Pras, A. Holden, E. Behr, A. Poduri; P. Lugar, D. Marchuk, S. Kerns, H. Oster, R. Gbadegesin, M. Winn, E.J. Holtzman, Y.-H. Jiang, R. Brown, S.H. Appel, E. Simpson, S. Halton, L. Lay, R. Bedlack, K. Grace. This work was funded in the Caldecott laboratory by the Medical Research Council (MRC; MR/J006750/1 and G0901606/1) and Cancer Research UK (C6563/A16771), in the Cortes-Ledesma laboratory by the Spanish government (SAF2010-21017, RYC-2009-03928 and JAE-Doc 2010-011) and European Union (PERG07-2010-268466), in the El-Khamisy laboratory by the Wellcome Trust (fellowship 085284 and grant 091043) and the Lister Institute of Preventative Medicine (fellowship), and in part by the Netherlands Organization for Health Research and Development (ZonMW; VIDI grant 917-86-319 to B.B.A.d.V.), the GENCODYS project (EU-7th-2010-241995 to B.B.A.d.V. and A.P.M.d.B.), a Brainwave–Irish Epilepsy Association/Medical Research Charities Group of Ireland/Health Research Board award (2009/001) and a Health Research Board of Ireland Translational Research Scholars award. Control samples were funded by National Institute for Mental Health (NIMH) awards (RC2MH089915, K01MH098126, R01MH099216 and R01MH097971), the Epi4K Gene Discovery in Epilepsy study (National Institute for Neurological Disorders and Stroke (NINDS) U01NS077303), the Epilepsy Genome/Phenome Project (EPGP; NINDS U01NS053998), the Center for HIV/AIDS Vaccine Immunology (CHAVI) study (National Institute of Allergy and Infectious Diseases (NIAID) UO1AIO67854), the Ellison Medical Foundation New Scholar award (AG-NS-0441-08), SAIC-Frederick, Inc. (M11-074) and Biogen Idec, Inc.
Author information
Authors and Affiliations
Contributions
K.W.C. devised and coordinated the project. K.W.C. and F.G.-H. designed and interpreted the biochemical, cell biology and mouse experiments and wrote the manuscript. F.G.-H. conducted all biochemical and cell biology experiments. S.R. analyzed mouse interneurons, and F.C.-L. and R.R.-G. measured TDP2 activity in mouse brain tissue. J.H.M.S.-H., A.P.M.d.B. and B.B.A.d.V. conducted and interpreted exome sequencing under the supervision of B.B.A.d.V. and identified the human splice-site mutation in Nijmegen. M.M., J.C., S.E. and G.L.C. conducted and interpreted genome-wide association study and homozygosity mapping in Ireland under the supervision of G.L.C. and identified the TDP2 splice-site mutation by exome sequencing in collaboration with the Duke Center for Human Genome Variation. E.C., N.D. and T.J.C. recruited and phenotyped patients in Ireland. M.T.G. consulted, phenotyped and liaised with patients and their families in Ireland. S.F.E.-K. identified and coordinated the analysis of the TDP2 patient in Egypt.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Regions of homozygosity (ROH) in patients with mutated TDP2.
a, Summary of ROHs of ≥1 Mb per individual. Phe, phenotype status (U, unaffected, A, affected); NSEG, number of ROH segments; Mb, total size of ROH; MbAVG, average size of each ROH. b, Visualisation of shared ROH segments in the UCSC Genome Browser among affected (red) and unaffected (blue) individuals. A 9.08-Mb region along chromosome 6 is the sole region unique to all three affected siblings.
Supplementary Figure 2 Putative splice-site mutation in TDP2.
a, Raw sequence reads from exome sequencing of inidividual IV-9 showing the homozygous c.425+1G>A mutation. The orientation of the genomic sequence is 5′ to 3′ and is the transcribed/template strand. b, Confirmation by Sanger sequencing of the homozygous mutation in individual IV-9, the heterozygous mutation in the mother, and the wild-type allele in an unrelated control individual. The orientation of the genomic sequence is 5′ to 3′ and is the transcribed/template strand. c, Predicted effect of the c.425+1G>A mutation on mRNA splicing. Scenario A involves retention of intron 3, resulting in the introduction of a premature stop codon in the p.Leu142fs* alteration. Scenario B involves skipping of exon 3, resulting in the introduction of a premature stop codon in the p.Tyr84* alteration. Scenario C involves the use of an alternative splice-donor site resulting in the frameshift p.Gly135fs*16.
Supplementary Figure 3 Evolutionary conservation of TDP2.
Alignment (CLUSTALW) of TDP2 orthologs (frog, Xenopus tropicalis; chick, Gallus gallus; worm, Caenorhabditis elegans; zebrafish, Danio rerio). Identical residues are indicated by blue boxes, and conserved motifs of the metal-dependent phosphodiesterase superfamily are indicated by red boxes. Catalytic residues are indicated by yellow ovals. The three consequences of the putative splice-site mutation in the Irish patients are indicated in red, and the consequence of the dinucleotide substitution in the Egyptian patient is indicated in green.
Supplementary Figure 4 Impact of the TDP2 splice-site mutation (c.425+1G>A) on TDP2 mRNA.
a, Impact of the splice-site mutation on TDP2 mRNA size, as measured by non-quantitative RT-PCR amplification of TDP2 exons 1–6 in RNA from lymphoblastoid cells of the three affected individuals (IV-9, IV-14, IV-16) and an unrelated control individual (NR). Primer sequences are indicated in Supplementary Table 5. b, Impact of the splice-site mutation on TDP2 mRNA levels as measured by qRT-PCR. Data are the mean expression of TDP2 mRNA in all three affected individuals (A, n = 3) compared to the non-related control (NR, n = 8) in the absence (– CH) or presence (+ CH) of cycloheximide treatment to inhibit nonsense-mediated mRNA decay. Quantifications were performed in duplicate and were normalized against GUSB and PPIB levels.
Supplementary Figure 5 Absence of 5′-TDP activity in Irish and Egyptian patients harbouring homozygous truncation mutations in TDP2.
5′-TDP (left) and 3′-TDP (right) activity in protein extract from total blood from an unrelated control (UC) and from affected Irish (IV-9) or Egyptian (E) patients with independent TDP2 mutations. B, negative control lacking protein extract. The radioactively labeled strand of the substrate is indicated with an asterisk.
Supplementary Figure 6 TDP2 mRNA expression in different fetal and adult human tissues.
Relative expression levels are given as the fold change in comparison to the tissue with the lowest expression level. Quantifications were performed in duplicate and normalized against GUSB and PPIB levels.
Supplementary Figure 7 Repair of TOP2-induced DSBs in mouse neural cells.
Decreased repair of TOP2-induced DSBs in Tdp2Δ1–3 mouse cortical astrocytes (top) and cerebellar granule neurons (bottom). Representative images are shown of etoposide-induced (20 mM) γH2AX immunofoci in Tdp2+/+ and in Tdp2Δ1–3 astrocytes and Tdp2+/δ1–3 and Tdp2Δ1–3 granule neurons after a 3-h recovery period in etoposide-free medium.
Supplementary Figure 8 Transcription of AR-responsive genes is inhibited by abortive TOP2 activity.
a, mRNA levels of three AR-responsive genes (KLK2, TMPRSS2 and KLK3) and three genes unresponsive to AR (ACT, TBP and MLN51) before and at the indicated times (h) after incubation with 100 nM DHT. mRNA levels were normalized to ACTIN and then made relative to the untreated time point (– DHT). b, Inhibition of AR-induced gene transcription by etoposide-induced abortive TOP2 activity. mRNA levels of the indicated AR-responsive (KLK2, TMPRSS2 and KLK3) and unresponsive (ACT, TBP and MLN51) genes before (– DHT) and after (+ DHT) stimulation with 100 nM DHT and after induction with DHT in the presence of the indicated concentration of etoposide. mRNA levels were quantified as described in the main text. c, Recruitment of RNAP II at the KLK3 and TMPRSS2 promoters in the absence of gene induction (– DHT), 8 h after gene induction with 100 nM DHT (+ DHT) and after gene induction with 100 μM etoposide present for two additional hours (+ DHT/+ Etop.). Data are presented as percentage of DNA precipitated (left) or relative to the recovery of a non-transcribed region of chromosome 5 (right) and are the mean (± s.e.m.) of at least three independent experiments.
Supplementary Figure 9 TDP2 depletion by RNA interference.
TDP2 depletion in LNCaP cells by short hairpin RNA (shRNA) (left) or siRNA (right). Cells employed to measure RNAP II promoter occupancy by chromatin immunoprecipitation (left) or AR-dependent gene expression by qRT-PCR (right) were subjected to protein blotting to measure the levels of the proteins indicated.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–6 and Supplementary Note (PDF 9936 kb)
Rights and permissions
About this article
Cite this article
Gómez-Herreros, F., Schuurs-Hoeijmakers, J., McCormack, M. et al. TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nat Genet 46, 516–521 (2014). https://doi.org/10.1038/ng.2929
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2929
This article is cited by
-
A novel pathogenic variant in TDP2 causes spinocerebellar ataxia autosomal recessive 23 accompanied by pituitary tumor and hyperhidrosis: a case report
Neurological Sciences (2024)
-
Inactivating TDP2 missense mutation in siblings with congenital abnormalities reminiscent of fanconi anemia
Human Genetics (2023)
-
The threat of programmed DNA damage to neuronal genome integrity and plasticity
Nature Genetics (2022)
-
In vivo 5-ethynyluridine (EU) labelling detects reduced transcription in Purkinje cell degeneration mouse mutants, but can itself induce neurodegeneration
Acta Neuropathologica Communications (2021)
-
ATR regulates neuronal activity by modulating presynaptic firing
Nature Communications (2021)