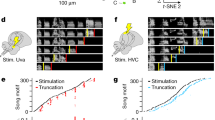
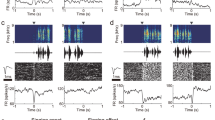
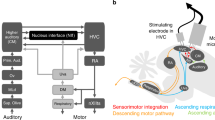
Abstract
Songbirds learn precisely sequenced motor skills (songs) subserved by distinct brain areas, including the premotor cortical analog HVC, which is essential for producing learned song, and a 'cortical'–basal ganglia loop required for song plasticity. Inputs from these nuclei converge in RA (robust nucleus of the arcopallium), making it a likely locus for song learning. However, activity-dependent synaptic plasticity has never been described in either input. Using a slice preparation, we found that stimulation patterns based on singing-related activity were able to drive opposing changes in the strength of RA's inputs: when one input was potentiated, the other was depressed, with the direction and magnitude of changes depending on the relative timing of stimulation of the inputs. Moreover, pharmacological manipulations that blocked synaptic plasticity in vitro also prevented reinforcement-driven changes to song in vivo. Together, these findings highlight the importance of precise timing in the basal ganglia–motor cortical interactions subserving adaptive motor skills.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nordeen, K.W. & Nordeen, E.J. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav. Neural Biol. 57, 58–66 (1992).
Brainard, M.S. & Doupe, A.J. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404, 762–766 (2000).
Leonardo, A. & Konishi, M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature 399, 466–470 (1999).
Andalman, A.S. & Fee, M.S. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc. Natl. Acad. Sci. USA 106, 12518–12523 (2009).
Charlesworth, J.D., Warren, T.L. & Brainard, M.S. Covert skill learning in a cortical-basal ganglia circuit. Nature 486, 251–255 (2012).
Kojima, S., Kao, M.H. & Doupe, A.J. Task-related 'cortical' bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc. Natl. Acad. Sci. USA 110, 4756–4761 (2013).
Aronov, D., Andalman, A.S. & Fee, M.S. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320, 630–634 (2008).
Nottebohm, F., Stokes, T.M. & Leonard, C.M. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457–486 (1976).
Bottjer, S.W., Miesner, E.A. & Arnold, A.P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901–903 (1984).
Scharff, C. & Nottebohm, F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 11, 2896–2913 (1991).
Kao, M.H. & Brainard, M.S. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J. Neurophysiol. 96, 1441–1455 (2006).
Ölveczky, B.P., Andalman, A.S. & Fee, M.S. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 3, e153 (2005).
Sober, S.J., Wohlgemuth, M.J. & Brainard, M.S. Central contributions to acoustic variation in birdsong. J. Neurosci. 28, 10370–10379 (2008).
Thompson, J.A., Wu, W., Bertram, R. & Johnson, F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J. Neurosci. 27, 12308–12320 (2007).
Herrmann, K. & Arnold, A. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: a quantitative electron microscopic study. J. Neurosci. 11, 2063–2074 (1991).
Kittelberger, J.M. & Mooney, R. Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway. J. Neurosci. 19, 9385–9398 (1999).
Iyengar, S. & Bottjer, S.W. The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J. Neurosci. 22, 946–958 (2002).
Hahnloser, R.H.R., Kozhevnikov, A.A. & Fee, M.S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70 (2002).
Kao, M.H., Wright, B.D. & Doupe, A.J. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J. Neurosci. 28, 13232–13247 (2008).
Spiro, J.E., Dalva, M.B. & Mooney, R. Long-range inhibition within the zebra finch song nucleus RA can coordinate the firing of multiple projection neurons. J. Neurophysiol. 81, 3007–3020 (1999).
Mooney, R. & Konishi, M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc. Natl. Acad. Sci. USA 88, 4075–4079 (1991).
Stark, L.L. & Perkel, D.J. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J. Neurosci. 19, 9107–9116 (1999).
Cavara, N.A. & Hollmann, M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol. Neurobiol. 38, 16–26 (2008).
Warren, T.L., Tumer, E.C., Charlesworth, J.D. & Brainard, M.S. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J. Neurophysiol. 106, 1806–1821 (2011).
Tumer, E.C. & Brainard, M.S. Performance variability enables adaptive plasticity of 'crystallized' adult birdsong. Nature 450, 1240–1244 (2007).
Scott, L.L., Nordeen, E.J. & Nordeen, K.W. LMAN lesions prevent song degradation after deafening without reducing HVC neuron addition. Dev. Neurobiol. 67, 1407–1418 (2007).
Woolley, S.C., Rajan, R., Joshua, M. & Doupe, A.J. Emergence of context-dependent variability across a basal ganglia network. Neuron 82, 208–223 (2014).
Ali, F. et al. The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong. Neuron 80, 494–506 (2013).
Feldman, D.E. The spike-timing dependence of plasticity. Neuron 75, 556–571 (2012).
Fiete, I.R., Hahnloser, R.H.R., Fee, M.S. & Seung, H.S. Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. J. Neurophysiol. 92, 2274–2282 (2004).
Troyer, T.W. & Doupe, A.J. An associational model of birdsong sensorimotor learning I. Efference copy and the learning of song syllables. J. Neurophysiol. 84, 1204–1223 (2000).
Kullmann, D.M. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog. Brain Res. 125, 339–351 (2000).
Tamaru, Y., Nomura, S., Mizuno, N. & Shigemoto, R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106, 481–503 (2001).
Egger, V., Feldmeyer, D. & Sakmann, B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat. Neurosci. 2, 1098–1105 (1999).
Tyszkiewicz, J.P., Gu, Z., Wang, X., Cai, X. & Yan, Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C–dependent mechanism in pyramidal neurones of rat prefrontal cortex. J. Physiol. (Lond.) 554, 765–777 (2004).
Wada, K., Sakaguchi, H., Jarvis, E.D. & Hagiwara, M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J. Comp. Neurol. 476, 44–64 (2004).
Bell, C.C., Han, V.Z., Sugawara, Y. & Grant, K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387, 278–281 (1997).
Tzounopoulos, T., Kim, Y., Oertel, D. & Trussell, L.O. Cell-specific, spike timing–dependent plasticities in the dorsal cochlear nucleus. Nat. Neurosci. 7, 719–725 (2004).
Humeau, Y., Shaban, H., Bissière, S. & Lüthi, A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 426, 841–845 (2003).
Cho, J.-H. et al. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala. Nat. Neurosci. 15, 113–122 (2012).
Kimpo, R.R., Theunissen, F.E. & Doupe, A.J. Propagation of correlated activity through multiple stages of a neural circuit. J. Neurosci. 23, 5750–5761 (2003).
Giret, N., Kornfeld, J., Ganguli, S. & Hahnloser, R.H.R. Evidence for a causal inverse model in an avian cortico-basal ganglia circuit. Proc. Natl. Acad. Sci. USA 111, 6063–6068 (2014).
Kao, M.H., Doupe, A.J. & Brainard, M.S. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433, 638–643 (2005).
Mooney, R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J. Neurosci. 20, 5420–5436 (2000).
Leblois, A., Bodor, A.L., Person, A.L. & Perkel, D.J. Millisecond timescale disinhibition mediates fast information transmission through an avian basal ganglia loop. J. Neurosci. 29, 15420–15433 (2009).
Leblois, A., Wendel, B.J. & Perkel, D.J. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J. Neurosci. 30, 5730–5743 (2010).
Fujimoto, H., Hasegawa, T. & Watanabe, D. Neural coding of syntactic structure in learned vocalizations in the songbird. J. Neurosci. 31, 10023–10033 (2011).
Prather, J.F., Peters, S., Nowicki, S. & Mooney, R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451, 305–310 (2008).
Amador, A., Perl, Y.S., Mindlin, G.B. & Margoliash, D. Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature 495, 59–64 (2013).
Hanuschkin, A., Ganguli, S. & Hahnloser, R.H.R. A Hebbian learning rule gives rise to mirror neurons and links them to control theoretic inverse models. Front. Neural Circuits 7, 106 (2013).
Murugan, M., Harward, S., Scharff, C. & Mooney, R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron 80, 1464–1476 (2013).
Sizemore, M. & Perkel, D.J. Premotor synaptic plasticity limited to the critical period for song learning. Proc. Natl. Acad. Sci. USA 108, 17492–17497 (2011).
Stepanek, L. & Doupe, A.J. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J. Neurophysiol. 104, 2474–2486 (2010).
Acknowledgements
The authors would like to thank M. Brainard, M. Stryker, K. Bender, F. Fernandez, R. Nicoll, and members of the Doupe, Brainard and Sabes laboratories for discussions and comments on the manuscript, particularly the exceptional patience of M. Kao. This work was supported by US National Institutes of Health grant MH55987 and a NARSAD Distinguished Investigator award (A.J.D.), and Canadian Institute of Health Research and Grass Foundation Fellowships (W.H.M.).
Author information
Authors and Affiliations
Contributions
A.J.D. and W.H.M. designed the experiments and wrote the manuscript. W.H.M. conducted the experiments and analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Synaptic currents observed after LMAN stimulation are incompletely blocked at hyperpolarized potentials.
a) Stimulation of LMAN currents isolated with DNQX, and intracellular QX-314, D-890 and K-Methylsulfonate to inhibit AMPA, Na+, Ca2+, and K+ currents. Even at hyperpolarized potentials stimulation of LMAN axons reveals a DNQX insensitive, rectifying current. This current reverses slightly above 0mV (b,c) and shows voltage dependence, but is incompletely blocked at hyperpolarized potentials.
Supplementary Figure 2 Time Course of Stable Changes to Synaptic Strength.
a,b) Paired stimulation of LMAN and HVC can lead to stable changes in synaptic strength which could be observed out to 50 minutes (0 ms delay) and 35 minutes (-100 ms delay).
Supplementary Figure 3 GABAA mediated inhibition does not alter synaptic amplitude, timing, or plasticity and has little influence near choride reversal.
a) Gabazine has little to no effect on membrane potentials at -80 mV (a), but influences the membrane potentials at depolarized voltages (a, 0mV). Further, Gabazine does not seem to alter the peak timing or current of EPSCs at -80 mV (b,c, p < 0.05), likely due to the IPSCs occurring well after the EPSC peak (c). Further, the presence of GABAA blockers fails to prevent lasting plasticity after paired simultaneous stimulation (d).
Supplementary Figure 4 Application of mGlur2/3 agonist LY35470 causes synaptic changes similar to those seen during paired simultaneous stimulation.
a) LY354740 causes increased paired pulse ratio and synaptic depression in HVC, while causing an increase in synaptic strength in LMAN without altering paired pulse ratio (a,b). Further, LY35470 causes no significant change in the amplitude of minuature EPSCs, but lowers their frequency (c,d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 609 kb)
Rights and permissions
About this article
Cite this article
Mehaffey, W., Doupe, A. Naturalistic stimulation drives opposing heterosynaptic plasticity at two inputs to songbird cortex. Nat Neurosci 18, 1272–1280 (2015). https://doi.org/10.1038/nn.4078
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4078
This article is cited by
-
Vocal learning promotes patterned inhibitory connectivity
Nature Communications (2017)