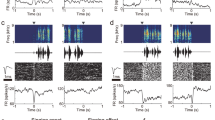
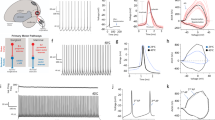
Abstract
While motor cortical circuits contain information related to specific movement parameters1, long-range inputs also have a critical role in action execution2,3. Thalamic projections can shape premotor activity2,3,4,5,6 and have been suggested7 to mediate the selection of short, stereotyped actions comprising more complex behaviours8. However, the mechanisms by which thalamus interacts with motor cortical circuits to execute such movement sequences remain unknown. Here we find that thalamic drive engages a specific subpopulation of premotor neurons within the zebra finch song nucleus HVC (proper name) and that these inputs are critical for the progression between vocal motor elements (that is, ‘syllables’). In vivo two-photon imaging of thalamic axons in HVC showed robust song-related activity, and online perturbations of thalamic function caused song to be truncated at syllable boundaries. We used thalamic stimulation to identify a sparse set of thalamically driven neurons within HVC, representing ~15% of the premotor neurons within that network. Unexpectedly, this population of putative thalamorecipient neurons is robustly active immediately preceding syllable onset, leading to the possibility that thalamic input can initiate individual song components through selectively targeting these ‘starter cells’. Our findings highlight the motor thalamus as a director of cortical dynamics in the context of an ethologically relevant behavioural sequence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Shenoy, K. V., Sahani, M. & Churchland, M. M. Cortical control of arm movements: a dynamical systems perspective. Annu. Rev. Neurosci. 36, 337–359 (2013).
Inagaki, H. K. et al. A midbrain–thalamus–cortex circuit reorganizes cortical dynamics to initiate movement. Cell 185, 1065–1081 (2022).
Dacre, J. et al. A cerebellar–thalamocortical pathway drives behavioral context-dependent movement initiation. Neuron 109, 2326–2338 (2021).
Guo, Z. V. et al. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186 (2017).
Gaidica, M., Hurst, A., Cyr, C. & Leventhal, D. K. Distinct populations of motor thalamic neurons encode action initiation, action selection, and movement vigor. J. Neurosci. 38, 6563–6573 (2018).
Sauerbrei, B. A. et al. Cortical pattern generation during dexterous movement is input-driven. Nature 577, 386–391 (2020).
Logiaco, L., Abbott, L. F. & Escola, S. Thalamic control of cortical dynamics in a model of flexible motor sequencing. Cell Rep. 35, 109090 (2021).
Tanji, J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu. Rev. Neurosci. 24, 631–651 (2001).
Glaze, C. M. & Troyer, T. W. Temporal structure in zebra finch song: implications for motor coding. J. Neurosci. 26, 991–1005 (2006).
Cynx, J. Experimental determination of a unit of song production in the zebra finch (Taeniopygia guttata). J. Comp. Psychol. 104, 3–10 (1990).
Okubo, T. S., Mackevicius, E. L., Payne, H. L., Lynch, G. F. & Fee, M. S. Growth and splitting of neural sequences in songbird vocal development. Nature 528, 352–357 (2015).
Coleman, M. J. & Vu, E. T. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J. Neurobiol. 63, 70–89 (2005).
Coleman, M. J., Roy, A., Wild, J. M. & Mooney, R. Thalamic gating of auditory responses in telencephalic song control nuclei. J. Neurosci. 27, 10024–10036 (2007).
Danish, H. H., Aronov, D. & Fee, M. S. Rhythmic syllable-related activity in a songbird motor thalamic nucleus necessary for learned vocalizations. PLoS ONE 12, e0169568 (2017).
Williams, H. & Vicario, D. S. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J. Neurobiol. 24, 903–912 (1993).
Elmaleh, M., Kranz, D., Asensio, A. C., Moll, F. W. & Long, M. A. Sleep replay reveals premotor circuit structure for a skilled behavior. Neuron 109, 3851–3861 (2021).
Nottebohm, F., Kelley, D. B. & Paton, J. A. Connections of vocal control nuclei in the canary telencephalon. J. Comp. Neurol. 207, 344–357 (1982).
Akutagawa, E. & Konishi, M. New brain pathways found in the vocal control system of a songbird. J. Comp. Neurol. 518, 3086–3100 (2010).
Mooney, R. & Prather, J. F. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J. Neurosci. 25, 1952–1964 (2005).
Egger, R. et al. Local axonal conduction shapes the spatiotemporal properties of neural sequences. Cell 183, 537–548 (2020).
Kornfeld, J. et al. EM connectomics reveals axonal target variation in a sequence-generating network. eLife 6, e24364 (2017).
Long, M. A. & Fee, M. S. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456, 189–194 (2008).
Picardo, M. A. et al. Population-level representation of a temporal sequence underlying song production in the zebra finch. Neuron 90, 866–876 (2016).
Hahnloser, R. H., Kozhevnikov, A. A. & Fee, M. S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70 (2002).
Kozhevnikov, A. A. & Fee, M. S. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 97, 4271–4283 (2007).
Hamaguchi, K., Tanaka, M. & Mooney, R. A distributed recurrent network contributes to temporally precise vocalizations. Neuron 91, 680–693 (2016).
Andalman, A. S., Foerster, J. N. & Fee, M. S. Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. PLoS ONE 6, e25461 (2011).
Schmidt, M. F. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. J. Neurophysiol. 90, 3931–3949 (2003).
Valverde, S. et al. Deep brain stimulation-guided optogenetic rescue of parkinsonian symptoms. Nat. Commun. 11, 2388 (2020).
Lymer, J., Prescott, I. A. & Levy, R. Microstimulation-induced inhibition of thalamic reticular nucleus in non-human primates. Exp. Brain Res. 237, 1511–1520 (2019).
Arfin, S. K., Long, M. A., Fee, M. S. & Sarpeshkar, R. Wireless neural stimulation in freely behaving small animals. J. Neurophysiol. 102, 598–605 (2009).
Vu, E. T., Mazurek, M. E. & Kuo, Y. C. Identification of a forebrain motor programming network for the learned song of zebra finches. J. Neurosci. 14, 6924–6934 (1994).
Ashmore, R. C., Wild, J. M. & Schmidt, M. F. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J. Neurosci. 25, 8543–8554 (2005).
Roberts, T. F. et al. Identification of a motor-to-auditory pathway important for vocal learning. Nat. Neurosci. 20, 978–986 (2017).
Fee, M. S., Kozhevnikov, A. A. & Hahnloser, R. H. Neural mechanisms of vocal sequence generation in the songbird. Ann. NY Acad. Sci. 1016, 153–170 (2004).
Scharff, C., Kirn, J. R., Grossman, M., Macklis, J. D. & Nottebohm, F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25, 481–492 (2000).
Zhao, W., Garcia-Oscos, F., Dinh, D. & Roberts, T. F. Inception of memories that guide vocal learning in the songbird. Science 366, 83–89 (2019).
Cardin, J. A., Raksin, J. N. & Schmidt, M. F. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J. Neurophysiol. 93, 2157–2166 (2005).
Otchy, T. M. et al. Acute off-target effects of neural circuit manipulations. Nature 528, 358–363 (2015).
Vyssotski, A. L., Stepien, A. E., Keller, G. B. & Hahnloser, R. H. A neural code that is isometric to vocal output and correlates with its sensory consequences. PLoS Biol. 14, e2000317 (2016).
Kosche, G., Vallentin, D. & Long, M. A. Interplay of inhibition and excitation shapes a premotor neural sequence. J. Neurosci. 35, 1217–1227 (2015).
Cannon, J., Kopell, N., Gardner, T. & Markowitz, J. Neural sequence generation using spatiotemporal patterns of inhibition. PLoS Comput. Biol. 11, e1004581 (2015).
Miri, A. et al. Spatial gradients and multidimensional dynamics in a neural integrator circuit. Nat. Neurosci. 14, 1150–1159 (2011).
Reinke, H. & Wild, J. M. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J. Comp. Neurol. 391, 147–163 (1998).
Schmidt, M. F. & Wild, J. M. The respiratory–vocal system of songbirds: anatomy, physiology, and neural control. Prog. Brain Res. 212, 297–335 (2014).
Johnson, M. D. & Ojemann, G. A. The role of the human thalamus in language and memory: evidence from electrophysiological studies. Brain Cogn. 42, 218–230 (2000).
Jurgens, U. Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 26, 235–258 (2002).
Pattinson, K. T. et al. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage 44, 295–305 (2009).
Wild, J. M. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J. Comp. Neurol. 349, 512–535 (1994).
During, D. N. et al. Fast retrograde access to projection neuron circuits underlying vocal learning in songbirds. Cell Rep. 33, 108364 (2020).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L. & Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440 (2010).
Kollmorgen, S., Hahnloser, R. H. R. & Mante, V. Nearest neighbours reveal fast and slow components of motor learning. Nature 577, 526–530 (2020).
Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M. & Kenneth, D. H. Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. Preprint at bioRxiv https://doi.org/10.1101/061481 (2016).
Rossant, C. et al. Spike sorting for large, dense electrode arrays. Nat. Neurosci. 19, 634–641 (2016).
Guizar-Sicairos, M., Thurman, S. T. & Fienup, J. R. Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158 (2008).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Katlowitz, K. A., Picardo, M. A. & Long, M. A. Stable sequential activity underlying the maintenance of a precisely executed skilled behavior. Neuron 98, 1133–1140 (2018).
Acknowledgements
We thank A. Banerjee, D. Jin, N. Nikbakht, A. Nieder, L. Veit, C. Bischof and members of the Long laboratory for comments on earlier versions of this manuscript. We thank J. Moore for technical advice on fluorescence quantification. A. Paulson provided technical assistance. This research was supported by R01 NS075044 (M.A.L.), F31 NS116933 (M.E.) and Simons Collaboration on the Global Brain (M.A.L.).
Author information
Authors and Affiliations
Contributions
F.W.M. and M.A.L. conceived the study and designed the experiments; F.W.M., M.E., L.A.A.-S., D.K. and A.C.A. conducted the research; F.W.M., M.E., L.A.A.-S. and M.A.L. performed data analyses; F.W.M., L.A.A.-S., A.C.A. and M.A.L. created the figures; F.W.M. and M.A.L. wrote the initial draft of the manuscript; F.W.M., M.E., A.C.A. and M.A.L. edited and reviewed the final manuscript. M.E. and M.A.L. acquired funding; M.A.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Melissa Coleman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Uva lesions impact syllabic song structure.
a,b, Silhouette criteria calculated on the prelesion (a) and postlesion (b) vocalizations for each bird (n = 8) for k-means of various sizes embedded using the first five PCs. Black circles superimposed on curves indicate the number of syllables in each bird’s song motif. c,d, Histology of Uva lesioned (n = 6) (c) and sham lesioned (n = 2) (d) birds (brightfield images, sagittal slices). White arrows indicate the extent of the lesions. CP: Posterior commissure, OM: occipitomesencephalic tract, SpM: nucleus spiriformis medialis, Cb: cerebellum. e, Schematic of the zebra finch brain at the medio-lateral level of Uva (sagittal view) based on an established anatomical resource (http://www.zebrafinchatlas.org/). The highlighted region is used in (f) and (g) to show the lesion extent (in transparent blue) for individual birds. f, g, t-SNE plots representing vocalizations taken from Uva lesioned (f) and sham lesioned (g) birds (prelesion green, postlesion black). Example sonograms depict pre- and postlesion song motifs.
Extended Data Fig. 2 Circuit-level consequences of electrical stimulation.
a,b, Example spike rasters (top) and peristimulus time histograms (bottom) for 6 HVC neurons that respond transiently (a) or are suppressed (b) by local high-frequency stimulation (see Methods). Red line indicates the onset of stimulation. Intensity and timing of stimulation identical to that used in behavioral experiments (i.e., Fig. 1). Inset: Average waveform for each neuron shows a variety of spike widths (Spike rise times - Cell a: 0.09 ms, Cell b: 0.34 ms, Cell c: 0.20 ms, Cell i: 0.36 ms, Cell ii: 0.41 ms, Cell iii: 0.30 ms. c, Activity profiles of 64 neurons recorded during local stimulation from 2 birds (bin size: 25 ms). At right, a closer view of responses from transiently active neurons (bin size: 5 ms). Color scale ranges from 0 (no firing) to 1 (maximum firing rate during observation period). d, Histogram showing the spike rise times (see Methods) for all recorded neurons. Transiently active neurons (red) often displayed shorter rise times, indicative of inhibitory interneurons.
Extended Data Fig. 3 Stimulation-related song truncation times across all birds.
a, Histology was used to confirm the placement of our stimulating electrodes (n = 10 birds). Shown here is an example bird in which we labeled upstream nuclei labeled with a retrograde GFP virus into HVC (n = 3 birds), including nucleus Uvaeformis (Uva) and nucleus interfacialis of the nidopallium (NIf). Arrows indicate the bipolar electrode tract (left) and the axonal tract emanating from Uva (right). b, Photography of bipolar stimulation electrode. c,d, Magnified views of Uva from the bird shown in (a). In (d), the dotted white line indicates the electrode tract and the diffuse green fluorescence nearby are small electrolytic lesions made to identify the electrode site. e–k, Stimulation (black ticks) and song truncation times (Uva: red ticks; HVC: blue ticks) for all stimulated motifs aligned to sonogram shown on top (E: 325 trials, F: 119 trials, G: 114 trials, H: 187 trials, I: 331 trials, J: 163 trials, K: 214 trials). For each plot, a histogram (bin size: 10 ms) shows the probability of a stimulation event and song truncation to occur (stimulation = black, Uva truncation: red, HVC truncation: blue). Orange bars indicate examples of long duration, complex syllables. l, Example songs truncated by unilateral Uva stimulation in either the left or the right hemisphere (yellow lines = stimulation times, red lines = song truncation times). Reference song at top. m, Proportion of song truncations related to closest syllable offset for unilateral stimulation (bird 156, left hemisphere: 86 trials; bird 156, right hemisphere: 64 trials; bird 471, right hemisphere: 32 trials).
Extended Data Fig. 4 Quantifying the population of HVC-projecting Uva neurons.
a–c, Sagittal view of HVC (white dotted line) into which retrograde tracers were injected (right is anterior) (n = 3 birds). NeuN labeled neurons shown in orange (a). Extent of fluorescently labeled cholera toxin subunit B (red) relative to HVC (b). Local labeling of cells with EGFP (green) after the injection of a retrograde virus into HVC (c). d–f, Spheres indicate retrogradely labeled HVC-projecting Uva neurons from three zebra finches shown in parasagittal (d), coronal (e), and transverse (f) views. Scale bars: 200 µm.
Extended Data Fig. 5 Uva-stimulation responses in each HVC cell type.
a–c, Activation ratios following Uva stimulation for premotor neurons (HVCRA) (a), basal ganglia-projecting neurons (HVCX) (b), and putative interneurons (HVCInt) and Avalanche-projecting neurons (HVCAv) (c). Left histogram same data as plots at right but on a logarithmic axis. d, Individual (gray) and average (red) fluorescence changes following Uva stimulation for each Uva driven HVCRA neuron from Fig. 3g.
Extended Data Fig. 6 UvD and nUvD spiking event times relative to song for premotor neurons across all birds.
a–f, All spiking event times for UvD and nUvD neurons across all birds aligned to sonogram and amplitude of song motif. Bird 490 (UvD = 11, nUvD = 58) (a), Bird 471 (UvD = 1, nUvD = 13) (b), Bird 513 (UvD = 10, nUvD = 30) (c), Bird 520 (UvD = 6, nUvD = 51) (d), Bird 486 (UvD = 2, nUvD = 1) (e), Bird 467 (UvD = 0, nUvD = 21) (f). g, Comparison of song amplitude following UvD and nUvD spiking events. nUvD (black) and UvD (red) lines show the median song amplitude following spiking events for recorded data (window: 4 to 19 ms post spiking event; nUvD, n = 174, UvD, n = 30) compared with a 99% confidence interval (CI) based on the distribution of 100,000 median song amplitude values (same window) drawn from data matched numbers of uniformly distributed, random event times (see Methods).
Extended Data Fig. 7 Spiking event times of UvD and nUvD HVCRA and HVCX neurons aligned to vocalization onset and offset.
a, Spiking event times of HVCRA neurons active during singing. Left: Aligned to the closest syllable onset (UvD, n = 23; nUvD, n = 106). Right: Aligned to the closest syllable offset (UvD, n = 19; nUvD, n = 89). b, Spiking event times of HVCRA neurons active during introductory notes, distance calls, or tet calls. Left: Aligned to the closest vocalization onset (UvD, n = 8, nUvD, n = 19). Right: Aligned to the closest vocalization offset (UvD, n = 0, nUvD n = 11). No spiking events from UvD HVCRA neurons were found within 50 ms of introductory note or call offsets. c, Left: Scatterplot displaying activation ratios and Uva stimulation evoked ΔF/F for 227 HVCRA neurons observed in the ketamine condition. Filled circles in the UvD cluster (red) were also classified as UvD cells in the awake condition shown in (d). Middle: Spiking event times of HVCRA neurons – classified based on the ketamine condition – aligned to the closest syllable onset (UvD, n = 29; nUvD, n = 114). Right: Aligned to the closest syllable offset (UvD, n = 18; nUvD, n = 90). d, Left: Same as (c) for 220 HVCRA neurons observed in the awake condition. Filled circles in the UvD cluster (red) were also classified as UvD cells in the ketamine condition shown in (c). Middle: Spiking event times of HVCRA neurons – classified based on the awake condition – aligned to the closest syllable onset (UvD, n = 24; nUvD, n = 113). Right: Aligned to the closest syllable offset (UvD, n = 13; nUvD, n = 88). e, Left: Scatterplot displaying activation ratios and Uva stimulation evoked ΔF/F for 667 HVCX neurons. Neurons were classified using either k-means clustering (top) or a ‘strict’ activation ratio threshold (bottom), which was set to 0.38 (i.e. the lowest activation ratio of the UvD cluster in Fig. 3g). Middle: Spiking event times of UvD HVC neurons aligned to the closest syllable onset (k-means: HVCRA, n = 31; HVCX, n = 50; strict: HVCX, n = 28). Right: Aligned to the closest syllable offset (k-means: HVCRA, n = 19; HVCX, n = 23, strict: HVCX, n = 13). Horizontal red, green or blue lines indicate moments in which the population exceeds a 99% CI of a uniform distribution.
Extended Data Fig. 8 Uva driven (UvD) premotor neurons active at motif and bout transition points.
a, Average fluorescence responses (± SEM) to Uva stimulation for 5 functionally defined UvD HVC neurons. Range: 23–26 stimulation trials. b, Activity of UvD neurons during singing. Song amplitude shown at top; fluorescence trace in red (scale bar = 2 ΔF/F). Individual song motifs indicated by green background shading. c, Song motif aligned fluorescence responses for all songs during which Cell #1 (n = 42 trials), Cell #70 (n = 15 trials), Cell #8 (n = 65 trials), Cell # 85 (n = 23 trials), and Cell #117 (n = 24 trials) were recorded. Black dotted line indicates the median spiking event time estimated across all fluorescence traces (see Methods). Traces normalized by maximum value.
Supplementary information
Supplementary Video 1
HVC activity during song. Imaged neurons expressing GCaMP6 show consistent activity during the performance of six repeated song motifs. Reponses are shown in real time. Neurons projecting to Area X are labelled with a red retrograde tracer (Methods).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moll, F.W., Kranz, D., Corredera Asensio, A. et al. Thalamus drives vocal onsets in the zebra finch courtship song. Nature 616, 132–136 (2023). https://doi.org/10.1038/s41586-023-05818-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05818-x
This article is cited by
-
Thalamic drive starts action sequences
Nature Reviews Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.