Abstract
The transcriptional repressor Bcl-6 is linked to the development of both CD4+ T follicular helper (TFH) and central memory T (TCM) cells. Here, we demonstrate that in response to decreased IL-2 signalling, T helper 1 (TH1) cells upregulate Bcl-6 and co-initiate TFH- and TCM-like gene programs, including expression of the cytokine receptors IL-6Rα and IL-7R. Exposure of this potentially bi-potent cell population to IL-6 favours the TFH gene program, whereas IL-7 signalling represses TFH-associated genes including Bcl6 and Cxcr5, but not the TCM-related genes Klf2 and Sell. Mechanistically, IL-7-dependent activation of STAT5 contributes to Bcl-6 repression. Importantly, antigen-specific IL-6Rα+IL-7R+ CD4+ T cells emerge from the effector population at late time points post influenza infection. These data support a novel role for IL-7 in the repression of the TFH gene program and evoke a divergent regulatory mechanism by which post-effector TH1 cells may contribute to long-term cell-mediated and humoral immunity.
Similar content being viewed by others
Introduction
During the course of an immune response, CD4+ T helper cells identify invading pathogens, proliferate and secrete cytokines to aid in immune-mediated clearance of infection. This results in an initial expansion of effector CD4+ T cells. As pathogen is eliminated, the number of CD4+ T cells is reduced to avoid potential autoimmunity that may result from a prolonged effector T-cell response. During this contraction phase, long-lived CD4+ T cells—termed memory T cells—survive to respond more quickly and robustly should the immune system re-encounter the same pathogen. The development of comprehensive immunological memory requires the generation of cells that are capable of contributing to both long-term cell-mediated and humoral immune responses, and includes populations of both CD4+ central memory T (TCM) and T-follicular helper (TFH) cells.
TCM cells are one of many CD4+ memory T-cell populations that also include effector memory (TEM), resident memory (TRM) and recirculating memory (TRCM) cell types1,2,3. These diverse sets of memory cells have been primarily identified by their immune function, tissue location and cell surface receptor expression. Specifically, TCM cells are uniquely identified by the expression of the homing receptors L-selectin (CD62L, encoded by the gene Sell) and Ccr7 (ref. 1). The expression of these two receptors allows for trafficking to the T-cell zones of secondary lymphoid areas where TCM cells can participate in cell-mediated immunity by engaging in antigen surveillance.
TFH cells facilitate humoral immune responses by assisting B-lymphocytes with the production of pathogen-neutralizing antibodies4,5,6. TFH cells are defined in part by the expression of the cell surface receptor Cxcr5, which allows for homing to the B cell zones of secondary lymphoid organs4,5. Differentiation of the TFH cell subset is a complex and multi-step process. These steps include the initiation of the TFH gene program, denoted by an initial upregulation of a partial TFH-profile (that is, Bcl-6 and Cxcr5 expression), followed by a second stage, whereby full commitment to the germinal center TFH cell state occurs upon B-cell interaction and enhanced ICOS signalling7. Given the inherent complexity of this process, many questions remain regarding the genesis of TFH cell populations. For example, while TFH cells can and do develop during the initial response to antigen, it has also been demonstrated that other effector T-helper cell subsets are capable of adopting a TFH-like profile, making a ‘post-effector’ developmental pathway plausible as well8,9,10,11,12,13. These previous studies are important as they support the possibility that, in addition to effector TFH cells, other T-helper populations may assist in long-term antibody-mediated immunity by co-opting certain aspects of the TFH-cell gene program.
It has been previously demonstrated that the transcriptional repressor Bcl-6 is required for TFH development14,15,16. Bcl-6 directs TFH differentiation, at least in part, by antagonizing the expression of a second transcriptional repressor, Blimp-1, a known negative regulator of TFH cell differentiation12,14,15,16. Interestingly, upregulation of Bcl-6 has also been implicated in the differentiation of CD4+ effector T cells into memory cells, including the TCM subset17,18,19,20. However, it is currently unclear how this increase in Bcl-6 expression initiates this effector-to-memory transition. As with TFH differentiation, it has been postulated that a key function of Bcl-6 in promoting memory cell formation is to repress the expression of Blimp-1, a factor positively linked to the terminal differentiation of the effector cell state2,21,22,23. However, whether there are additional roles or long-term requirements for Bcl-6 expression in establishing TCM-cell fate apart from Blimp-1 repression remains unclear.
Although transcription factors such as Bcl-6 are responsible for regulating the gene expression profiles of developing cells, upstream environmental signals, often in the form of a cytokine-dependent response, regulate the expression and/or the functional activity of individual transcription factors24. For example, it has been demonstrated that strong interleukin-2 (IL-2) signalling inhibits TFH formation by controlling the expression of Bcl-6 and Blimp-1 (refs 12, 25, 26, 27). Similarly, it has been shown that inflammatory cytokine environments, including those that are IL-2 rich, can inhibit memory cell formation28,29.
Collectively, evidence in the literature suggests that, despite their distinct functions and localization during the adaptive immune response, TFH and TCM cells share specific regulatory requirements and developmental programs. In addition, several recent studies have reported a high degree of similarity between TFH and TCM cells at the transcriptional and protein expression levels9,17,19,30. Furthermore, populations of memory TFH and TCM cells similarly express a number of cell surface receptors including Cxcr5, CD62L and Ccr7 (refs 8, 9, 30, 31, 32). Thus, there are precedents supporting the possibility that these two immune cell populations may be developmentally linked. However, as the contribution of each of these cells to long-term immunity is functionally distinct, it is likely that at some point there is a divergence to unique cytokine and transcriptional networks to allow for specialized TFH- and TCM-dependent immune responses.
Here, we demonstrate that T-helper 1 (TH1) cells are capable of co-initiating the expression of both TFH and TCM gene programs in response to decreased IL-2 signalling. Mechanistically, the initial co-expression of the TFH and TCM programs is due to the Bcl-6-dependent repression of Blimp-1. In addition, during this post-effector stage, TH1 cells downregulate IL-2Rα expression, while upregulating the dual expression of IL-6Rα and IL-7R. This apparent cytokine receptor reprogramming results in a population of bi-potent ‘TFH/TCM-like’ cells with the ability to respond to either IL-6 or IL-7. Strikingly, while treatment with IL-6 results in the upregulation of Bcl-6 and Cxcr5 expression, exposure of these cells to IL-7 results in a dose-dependent decrease in TFH-associated genes including Bcl-6, while allowing the continued expression of TCM-specific genes. Importantly, at late time points post influenza infection, a population of antigen-specific IL-6Rα+IL-7R+ CD4+ T cells emerges coincident with a decline in the effector population. Thus, this study describes a novel role for IL-7 in the repression of the TFH gene program and defines a potential mechanism by which post-effector TH1 cells may be able to support aspects of both long-term cell-mediated and humoral immunity.
Results
IL-2 signalling modulates TH1, TFH and TCM gene expression
Our previous studies demonstrated that reduced IL-2 signalling has the potential to regulate T-helper cell fate decisions by augmenting the expression of the TFH lineage-defining factor Bcl-6 (ref. 12). In addition to its required role in TFH cell development, Bcl-6 expression has also been implicated in the formation of memory CD4+ T cells1,17,19,20. As such, we examined whether the IL-2-sensitive increase in Bcl-6 expression would similarly result in the upregulation of a memory cell gene program by exposing in vitro generated TH1 cells to both high and low IL-2 concentrations. As with our prior study, the expression of a number of TH1 genes was decreased in low environmental IL-2 conditions, while the expression of key TFH genes was induced, including the expression of Bcl6 and Cxcr5 (Fig. 1a,b). Consistent with the increase in transcript, Bcl-6 protein and cell surface expression of Cxcr5 were significantly increased (Fig. 1c,d). Interestingly, in addition to the induction of the TFH-like profile, TH1 cells exposed to a low IL-2 concentration also upregulated genes associated with the TCM cell type—most notably the lymph node homing receptors Sell and Ccr7 (Fig. 1e)1,2,33. We also observed increased expression of other memory T-cell-related markers (Il7r, S1pr1, Cd27 and Cxcr3) and transcription factors known to promote the memory T-cell fate (Klf2, Klf3 and Foxo1; Fig. 1e,f). Interestingly, the expression of Tcf7, the gene that encodes the transcriptional regulator TCF-1, was significantly increased (Fig. 1f). Similar to Bcl-6, the expression of TCF-1 has been implicated in the development of both TFH and memory cells34,35,36,37,38. Importantly, the induction of the TCM-like profile was not limited to changes in transcript alone, as the cell surface expression of both CD62L and Ccr7 was significantly increased compared with that observed in effector TH1 cells (Fig. 1g,h). Collectively, these data demonstrate that increased Bcl-6 expression in TH1 cells, in response to decreased IL-2 signalling, results in the induction of not only a TFH-like profile, but a TCM-like profile as well. Furthermore, these data suggest that a common Bcl-6-dependent regulatory mechanism may be responsible for the initiation of both the TFH and TCM gene programs.
Primary CD4+ T cells were cultured in TH1 conditions and exposed to either high (TH1 cells) or low (TFH-like cells) environmental IL-2 (250 U ml−1 or 10 U ml−1, respectively). (a,b) RNA was isolated from the TH1 (white bar) and TFH-like (black bar) cells and the expression of the indicated genes was determined by quantitative RT–PCR. Data were normalized to Rps18 as a control and the results are represented as fold change in expression relative to the TH1 sample (mean of n=3±s.e.m.). (c) An immunoblot analysis was performed to assess changes in protein expression in response to alterations of environmental IL-2. Expression for Bcl-6 and Blimp-1 was measured with β-actin serving as a control for equal protein loading. Shown is a representative blot of three independent experiments performed. (d) Representative histogram overlay of cell surface expression of Cxcr5 for TH1 and TFH-like cells. Geometric mean fluorescence intensity (geoMFI) for Cxcr5 is also shown (mean of n=5±s.e.m.). (e,f) qRT–PCR analysis examining expression of the indicated genes in TH1 and TFH-like cells. Data were normalized and represented as in a and b (mean of n=3±s.e.m.). (g,h) Representative histogram overlay of cell surface expression of (g) CD62L and (h) Ccr7 for TH1 and TFH-like cells. Average geoMFI or MFI for CD62L and Ccr7 expression are shown (mean of n=5 or 4±s.e.m.). *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t-test).
Blimp-1 inhibits the TCM-associated genes Sell and Ccr7
We previously identified Blimp-1 as a factor responsible for directly repressing members of the TFH gene program including Cxcr5 and Il6ra12. A key finding from our prior studies was that in response to reduced IL-2 signalling, Bcl-6 expression increased in TH1 cells, resulting in the Bcl-6-dependent repression of Blimp-1 and the subsequent induction of the TFH gene program (Fig. 1c)12,39. Given our finding that increased Bcl-6 expression also leads to the induction of a TCM-like profile, we hypothesized that the same mechanistic interplay between Bcl-6 and Blimp-1 that results in the promotion of the TFH program may also initiate the transition from an effector TH1 to a TCM-like cell state. Therefore, we examined whether TCM genes, similar to TFH genes, were direct Blimp-1 targets in effector TH1 cells. Using predictive transcription factor binding site software (Genomatix), we identified potentially functional Blimp-1 DNA-binding elements in the promoters of both Sell and Ccr7. To determine whether the predicted elements were functional, we prepared promoter–reporter constructs encompassing the predicted sites and performed luciferase reporter experiments in the presence and absence of overexpressed Blimp-1. Importantly, the expression of Blimp-1 resulted in a decrease in the promoter activity of both Sell and Ccr7 (Fig. 2a,b). As a control, there was no Blimp-1-mediated repression of a Tbx21-reporter, which lacks predicted Blimp-1 DNA-binding elements. To determine whether Blimp-1-mediated repression may be the result of direct DNA binding, we performed promoter–reporter experiments with a mutant Blimp-1 construct lacking the zinc finger DNA-binding domain (ΔZF). Although wild-type Blimp-1 readily repressed promoter activity, there was no repression with the Blimp-1ΔZF protein (Fig. 2c,d). As a further test of the functional nature of the predicted sites, a Sell-reporter construct lacking the Blimp-1 DNA-binding element (pGL3-SellΔBL1) was not repressed by Blimp-1 (Fig. 2e,f).
(a,c,e,f) EL4 T cells were transfected with the indicated promoter–reporter constructs in combination with a wild-type Blimp-1 expression vector, a Blimp-1 mutant incapable of binding to DNA (ΔZF), or an empty vector control (mean of n=3±s.e.m.). In e and f, EL4 T cells were transfected with either a wild-type Sell promoter–reporter or a Sell promoter–reporter lacking the predicted Blimp-1 DNA-binding elements (pGL3-SellΔBL1). Luciferase promoter–reporter values were normalized to a renilla control and expressed relative to the control sample for each experiment (mean of n=4±s.e.m.). (b,d) Wild-type and mutant Blimp-1 protein levels were measured by immunoblot analysis. Shown is a representative blot of three independent experiments performed. (g,h) TH1 cells were nucleofected with either siRNA specific to Blimp-1 (siPrdm1) or a control siRNA. Following a 24-h time period, RNA was harvested and expression of Sell or Ccr7 was assessed by qRT–PCR. The data are presented as fold change in expression relative to the control sample (mean of n=4±s.e.m.). **P<0.01, ***P<0.001 (unpaired Student’s t-test). Cont., control; NS, not significant; WT, wild type.
To examine the extent of Blimp-1-specific repression in an endogenous setting, we utilized a knockdown approach to assess the effect of decreasing Blimp-1 levels on the expression of these genes in effector TH1 cells (Fig. 2g). Upon small interfering RNA (siRNA) knockdown of Blimp-1 expression, we observed a modest but significant increase in both Sell and Ccr7 expression (Fig. 2h). Collectively, these data suggest that the TCM-associated genes Sell and Ccr7 are repressed by Blimp-1 in effector TH1 cells. Furthermore, these findings suggest that the repression of Blimp-1 by Bcl-6 is a critical event for the initiation of both TFH and TCM gene programs in TH1 cells.
TH1 cells undergo cytokine receptor reprogramming
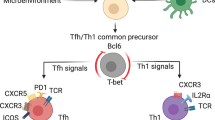
TCM and TFH cell differentiation represent complex- and multistep processes that are directed by a litany of factors. A key determinant that influences immune cell differentiation is the cytokine environment to which the cell is exposed, as well as the ability of that cell to sense and respond to its environment through cytokine receptor expression. Our data indicate that effector TH1 cells upregulate both TCM- and TFH-like gene expression patterns in response to a low IL-2 environment. Importantly, IL-2-signalling is known to influence the expression of cytokine receptors40. Initially, Il2ra is expressed at high levels in the effector TH1 cells. However, as these cells transition to a low IL-2 environment, our data demonstrate that Il2ra expression decreases, whereas the expression of Il6ra and Il7r increases (Fig. 1a,b,e). Hence, the predominant cytokine receptor expression pattern changes from one supportive of effector TH1 cells, which are responsive to elevated IL-2, to one enriched with IL-6Rα and IL-7R—receptors that respond to cytokines favouring TFH and TCM development, respectively41,42,43,44,45,46. Furthermore, these data are suggestive of the intriguing possibility that three divergent cell types may emerge from the effector TH1 population: a pre-TFH-like population (IL-6Rα+IL-7R−), a pre-TCM-like population (IL-6Rα−IL-7R+) and/or a bi-potent pre-TFH/TCM (IL-6Rα+IL-7R+) population that may be capable of transitioning into either cell type.
To address the above possibilities, we assessed the composition of both the high IL-2 and low IL-2- treated TH1 populations by examining the cell surface expression of IL-6Rα and IL-7R. Consistent with our transcript analysis, the expression of both IL-6Rα and IL-7R was significantly upregulated, whereas IL-2Rα was downregulated, on the surface of the low IL-2-treated cells (Fig. 3a–c). Importantly, the majority of the low IL-2-treated cells displayed dual expression of these receptors (double positive ‘DP’, IL-6Rα+IL-7R+), whereas comparably few of the DP cells were observed in the high IL-2-treated (TH1) population (Fig. 3d,e).
Primary CD4+ T cells were cultured in TH1 conditions and exposed to either high (TH1 cells) or low (TFH-like cells) environmental IL-2 (250 U ml−1 or 10 U ml−1, respectively). (a–e) Cell surface expression of IL-6Rα, IL-7R or IL-2Rα was measured by flow cytometric analysis. Data are represented as histograms (a–c), flow cytometry dot plots (d) or quantified percent positive cells (e). Average mean fluorescence intensity (MFI) for IL-6Rα, IL-7R and IL-2Rα expression is shown (mean of n=3±s.e.m.). **P<0.01, ***P<0.001 (unpaired Student’s t-test). DN, double negative; NS, not significant; SP, single positive.
To confirm that the IL-6Rα+IL-7R+ cells expressed both TFH-like and TCM-like programs, we sorted the DP population and compared the expression of key TH1, TFH and TCM genes to that observed in effector high IL-2-treated (TH1) and bulk low IL-2-treated (TFH-like) cells. Indeed, while significant differences were observed between the IL-6Rα+IL-7R+ DP and effector TH1 cells, the gene expression programs between sorted DP and bulk TFH-like cells were relatively indistinguishable (Fig. 4). Consistent with the transcript analysis, IL-6Rα+IL-7R+ cells also displayed elevated cell surface expression of CD62L, Ccr7 and Cxcr5 (Supplementary Fig. 1a–c). Collectively, these data support a model whereby, in response to reduced IL-2 signalling, TH1 cells co-initiate the expression of both TFH and TCM-like gene programs, including the dual expression of IL-6Rα and IL-7R (hereafter referred to as ‘TFH/TCM-like’ cells).
Droplet digital PCR analysis was used to examine expression of TH1-, TFH- and TCM-associated genes. IL-6Rα+IL-7R+ double-positive cells were sorted and their expression profiles were compared with that of TH1 (High IL-2) and TFH/TCM-like (Low IL-2) cells. Data were normalized to Rps18 and are represented as fold change in expression relative to the TH1 sample (mean of n=4±s.e.m.). *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t-test).
IL-6 and IL-7 differentially regulate TFH and TCM genes
The cytokines IL-6 and IL-7 have demonstrated roles in establishing and sustaining the TFH and TCM cell fates, respectively41,42,43,44,45,46. Therefore, to test the functional nature of IL-6Rα and IL-7R co-expression, we treated the TFH/TCM-like cells with either IL-6 or IL-7 and examined the expression of key TFH- and TCM-associated genes as compared with untreated controls. IL-6 treatment resulted in increased expression of the hallmark TFH genes Bcl6 and Cxcr5, but did not significantly impact the expression of the TCM gene Sell (Supplementary Fig. 2a,b). Conversely, exposure to IL-7 resulted in a significant reduction in Bcl-6 expression and the repression of several other TFH genes (Cxcr5, Il6ra, Sh2d1a and Cd40lg) (Fig. 5a,b). In many cases, the repression of the TFH-associated genes resulted in expression levels near to those observed in effector TH1 cells.
Primary CD4+ T cells were cultured in TH1 conditions and exposed to either high or low environmental IL-2 to generate effector TH1 or TFH/TCM-like (IL-6Rα+IL-7R+) cells, respectively. TFH/TCM-like cells were then exposed to IL-7. Following a 24 or 48 h incubation, expression of the indicated genes was measured by (a) qRT–PCR, (b) immunoblot or (c,d) flow cytometric analysis. For a, the sample values are presented as fold change in expression relative to the TH1 sample for each independent experiment (mean of n=3±s.e.m.). For b, Bcl-6 protein was measured with β-actin serving as a loading control. Shown is a representative blot of three independent experiments performed. For c and d, data are represented either as histogram flow cytometry plots or quantified geometric mean fluorescence intensity (geoMFI; mean of n=5±s.e.m.). *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t-test). NS, not significant.
As IL-7 is present at low levels in secondary lymphoid tissues, we next exposed the TFH/TCM-like cells to a range of physiologically relevant IL-7 concentrations and analysed the expression of key TCM and TFH genes (Supplementary Fig. 3)46. Strikingly, even at an extremely low concentration of environmental IL-7, hallmark TFH genes (Bcl6, Cxcr5 and Il6ra) were preferentially repressed. In stark contrast, the expression of the TCM-associated genes Sell, Klf2 and Ccr7 was relatively unaffected by IL-7 treatment. Importantly, the expression of the anti-apoptotic gene, Bcl2, and the gene encoding the glycerol channel aquaporin 9, Aqp9, both of which are known to promote the long-term survival of memory cells, was induced in response to IL-7 (Supplementary Fig. 3)47,48.
To further examine IL-7-induced changes to TFH and TCM cell surface marker expression, we compared Cxcr5 and CD62L protein expression in untreated TFH/TCM-like cells to those exposed to IL-7. Consistent with our transcript data, IL-7 treatment resulted in a significant reduction in Cxcr5 cell surface expression (Fig. 5c). In contrast, exposure to IL-7 resulted in a modest increase in CD62L, again suggesting that the repressive effect of IL-7 is limited to the TFH profile (Fig. 5d). It is important to note that in spite of the observed decrease in Cxcr5 expression, a majority of the Cxcr5 expressed on the cell surface remained refractory to the IL-7 repressive effect. This was in contrast to the almost complete loss of Bcl-6 protein expression in response to IL-7 treatment. These data corroborate other studies demonstrating that Cxcr5 expression can occur independent of Bcl-6 expression13,49. Furthermore, these data are consistent with the moderate expression of Cxcr5 observed on TCM cells1,19,50.
IL-7-induced repression of Bcl-6 is independent of Blimp-1
The finding that IL-7 represses Bcl-6 expression has potentially far-reaching implications given the demonstrated roles for Bcl-6 in TFH development, TCM differentiation and in the regulation of cellular functions including metabolism and cell cycle progression14,15,16,20,39,51,52. As such, we sought to determine the identity of the transcription factor(s) responsible for directing IL-7-mediated repression of Bcl-6. There is a well-established inverse relationship between the transcriptional repressors Bcl-6 and Blimp-1 (refs 12, 14, 17, 53). As our data demonstrate that IL-7-signalling inhibits Bcl-6 expression, we considered the possibility that IL-7 treatment may lead to increased Blimp-1 expression. To test this possibility, we examined the level of Prdm1 (Blimp-1) expression in TH1 cells compared with that of TFH/TCM-like cells, and TFH/TCM-like cells exposed to IL-7 (Fig. 6a). Consistent with our previous results, TH1 cells displayed significantly elevated expression of Prdm1 transcript as compared with TFH/TCM-like cells12. Interestingly, despite increased activation of STAT5 (which has been positively linked to Blimp-1 expression in response to IL-2 signalling), no appreciable increase in Blimp-1 protein was observed in the TFH/TCM-like cells stimulated with IL-7 (Fig. 6b). These data indicate that Blimp-1 is unlikely to be the IL-7-responsive factor that represses Bcl-6 expression. Furthermore, these data suggest that the IL-7-treated TFH/TCM-like cells are not simply reverting to a short-lived effector TH1 phenotype (that is, possessing high Blimp-1 expression). Rather, it appears they are transitioning into cells with a gene program that more closely resembles that of long-lived TCM cells.
(a) qRT–PCR or (b) immunoblot analysis of Blimp-1 expression in TH1, TFH/TCM-like cells, and TFH/TCM-like cells exposed to IL-7. In a, data were normalized to Rps18 as a control and the results are presented relative to the TH1 sample (mean of n=4±s.e.m.). In b, protein expression was assessed using the indicated antibody. β-Actin was monitored as a control for equal protein loading. Shown is a representative blot of three independent experiments. (c) Immunoblot analysis of Bcl-6, phospho-STAT5 (p-STAT5) and STAT5 expression in primary TH1 cells, TFH/TCM-like cells and TFH/TCM-like cells exposed to IL-7. β-Actin was monitored to ensure equal protein loading. Shown is a representative blot of three independent experiments. (d) A schematic indicating the location of a predicted tetrameric STAT5 binding site in the promoter of the Bcl6 locus. PCR amplicons used in the chromatin immunoprecipitation (ChIP) analysis are indicated as ‘A’ and ‘B’. (e) ChIP assay to assess STAT5 association with the Bcl6 locus in response to IL-7-signalling. Data are represented as percent enrichment relative to a ‘total’ input sample (mean of n=3±s.e.m.). *P<0.05, ***P<0.001 (unpaired Student’s t-test). NS, not significant.
IL-7 regulates STAT5 association with the Bcl6 promoter
It is well established that the transcription factor STAT5 is activated downstream of IL-7 signalling46,54,55. Although STAT5 is known to function as a transcriptional activator, recent studies have described novel roles for STAT5 in the direct repression of gene expression12,56. To determine whether IL-7-signalling represses Bcl-6 expression through the activation of STAT5, we compared the activation state of STAT5 (p-STAT5) in effector TH1, TFH/TCM-like cells and TFH/TCM-like cells exposed to IL-7 (Fig. 6c). Cells treated with IL-7 displayed elevated levels of STAT5 phosphorylation similar to that observed in effector TH1 cells. In contrast, TFH/TCM-like cells had relatively low STAT5 activation. Importantly, the increased level of STAT5 activation in IL-7-treated cells correlated with decreased Bcl-6 expression.
Given the inverse relationship between activated STAT5 and Bcl-6 expression, we hypothesized that STAT5 could play a role in the IL-7-mediated repression of Bcl-6 by directly binding to and repressing the Bcl6 promoter. In support of this hypothesis, a recent report demonstrated that a DNA sequence-specific tetrameric STAT5 complex functions downstream of IL-7R-signalling to repress gene expression during B-cell differentiation56. We analysed the Bcl6 locus and identified a potential tetrameric STAT5 DNA-binding site in the promoter region (Fig. 6d). We then performed chromatin immunoprecipitation (ChIP) analyses to determine whether STAT5 associates with the Bcl6 promoter in response to IL-7. Indeed, we detected increased STAT5 binding at the Bcl6 locus in TFH/TCM-like cells exposed to IL-7 as compared with untreated TFH/TCM-like cells (Fig. 6e). Importantly, the highest levels of STAT5 association corresponded to the location of the predicted tetrameric STAT5 binding site (Fig. 6d,e). Collectively, these data support a role for IL-7-activated STAT5 in the direct repression of Bcl-6 expression.
IL-6Rα+IL-7R+ CD4+ T cells emerge post influenza infection
A key discovery from our in vitro experiments was that effector TH1 cells upregulate both TFH and TCM gene programs in response to withdrawal of IL-2. These conditions are consistent with the late stages of an immune response, as the pro-inflammatory cytokine environment wanes and the effector population transitions to a memory population capable of supporting both cell-mediated and humoral immunity. Intriguingly, we observed that effector TH1 cells undergo a period of cytokine receptor reprogramming in which they downregulate IL-2Rα, upregulate IL-6Rα and IL-7R, and initiate the expression of hallmark TFH and TCM genes. Therefore, to assess the kinetics of IL-6Rα and IL-7R expression in vivo, we infected mice with influenza (A/PR8/34; ‘PR8’) and monitored antigen-specific (nucleoprotein, ‘NP’-specific) CD4+ T cells at multiple time points post infection (days post infection, d.p.i.). Shortly after infection (9 d.p.i.), cells could be divided into roughly two populations: effector TFH (Bcl-6HICxcr5HI) and effector non-TFH (Bcl-6MIDCxcr5MID). At this time point, the majority of the non-TFH effector population expressed low levels of IL-7R. However, at later time points (30 and 60 d.p.i.), a substantial percentage of this population displayed increased expression of IL-7R (Fig. 7a). In comparison, TFH cells expressed relatively low levels of IL-7R, especially at late time points post infection (Supplementary Fig. 4). Strikingly, the same cells that displayed increased expression of IL-7R also expressed the highest levels of IL-6Rα (Fig. 7b). Importantly, the IL-6Rα+IL-7R+ DP population increased with time post infection, whereas effector TFH and non-TFH (IL-7RLO) populations decreased (Fig. 7c). Collectively, these data indicate that a population of long-lived (60 d.p.i.), antigen-specific IL6Rα+IL7R+ DP cells arises during the post-influenza-infection period. Furthermore, the dual expression of IL-6Rα and IL-7R, in addition to our in vitro data demonstrating the differential regulation of TFH and TCM gene programs by IL-6 and IL-7, suggest that the activation of these cytokine-signalling pathways will likely influence the functional capabilities of the IL-6Rα+IL-7R+ cells in vivo.
Mice were infected with influenza (PR8) and CD4+CD19−Foxp3− cells were analysed by flow cytometric analysis at the indicated day post infection. (a) Nucleoprotein (NP)-specific cells were isolated and sorted into effector TFH (Eff TFH) and non-TFH (Eff TH) populations. Non-TFH effector cells were then assessed for IL-7R expression and further classified as IL-7R low (IL-7RLO) or IL-7R high (IL-7RHI). (b) IL-6Rα expression of the indicated cell population was measured by flow cytometric analysis (geometric mean fluorescence intensity (geoMFI); mean of n=5±s.d.). (c) Percent NP-specific distribution of Eff TFH, TH IL-7RLO and TH IL-7RHI cells at the indicated time points (Day 7, d9, d15, d30, d60) post influenza infection (mean of n=5±s.d.). **P<0.01 (unpaired Student’s t-test).
Discussion
Following infection, the contraction of the effector response and the subsequent emergence of the memory cell population are vital to long-lasting immunity. However, the potential for memory populations to arise from the numerous CD4+ T-cell subsets, as well as the degree of plasticity that exists between these subsets, has complicated the identification of specific environmental signals and precise transcriptional networks that direct the effector-to-memory transition. For example, TFH and TCM cells appear to share developmental pathways, including requirements for the transcriptional repressor Bcl-6 and low levels of IL-2 signalling14,15,16,19,20. Interestingly, our data suggest that the TFH and TCM gene programs can co-initiate from a population of effector TH1 cells upon increased Bcl-6 expression in response to IL-2 withdrawal, resulting in a ‘TFH/TCM-like’ population. In agreement with these data, there is emerging evidence for a set of genes which are linked to both TFH and memory cell development, including Bcl6, Cxcr5, Il7r and Tcf7 (refs 9, 19, 34, 35, 36, 37, 38). In addition, these data are physiologically intuitive as IL-2 signalling decreases during the late stages of an immune response, coincident with the formation of memory cell populations. Indeed, both TFH and TCM cells are required post contraction to mediate long-lasting humoral- and cell-mediated immunity, respectively. Therefore, it is likely that, following the initial co-expression of the TFH and TCM gene programs, there are distinct environmental and transcriptional regulatory mechanisms that promote TFH versus TCM-cell-dependent immune responses.
In support of this divergence, we observed that TH1 cells downregulate IL-2Rα while upregulating both IL-6Rα and IL-7R in response to a reduction in IL-2 signalling. Thus, these cells appear to reprogramme their ability to respond to both a TCM-essential (IL-7) and a TFH-associated (IL-6) cytokine. Our data further demonstrate that IL-6 treatment of TFH/TCM-like cells results in the further augmentation of the TFH profile. However, IL-7 exposure results in an inhibition of TFH genes, including Bcl-6. These findings highlight a previously unappreciated role for IL-7 in the repression of the TFH gene program in post-effector TH1 cells. Importantly, they also suggest that Bcl-6 may only be required for the initiation of the TCM gene program, and that further developmental steps require a unique and undefined set of transcriptional regulators. Indeed, there are many reports in the literature describing a reduction in Bcl-6 expression in TCM cells at late time points post infection7,13,19,30,57. Furthermore, these findings are consistent with defined roles for Bcl-6 in the regulation of metabolism, cell cycle progression and apoptosis39,46,47,51,52,58,59. Thus, IL-7-dependent repression of Bcl-6 may ultimately be necessary to promote the unique sets of regulatory activities required for long-term memory cell survival.
Interestingly, while IL-6 treatment augmented TFH gene expression, it did not affect the expression of TCM genes in the TFH/TCM-like post-effector population. Thus, these cells express hallmark genes of both TFH and TCM-cell types and resemble TFH-memory cells50. Whether there is a factor akin to IL-7 responsible for repressing the TCM-profile and promoting the TFH cell fate is a question that remains to be answered. In support of the model proposed here and perhaps shedding light on this question, recent reports have highlighted the antagonistic nature of TFH-associated ICOS signalling and the TCM transcription factor, Klf2. In these studies, ICOS signalling-dependent repression of Klf2 was required for commitment to the TFH-cell fate60,61. Thus, in addition to IL-6 signalling, interactions between TFH/TCM-like cells and B cells, along with the corresponding ICOS stimulation, are likely required to antagonize the TCM gene program and specifically promote TFH-dependent immune responses.
Our data demonstrate that the IL-7-dependent repression of Bcl-6 is not a result of increased Blimp-1 expression. This is surprising, as these transcriptional repressors often appear in opposition during developmental steps in the immune system. Rather, our current data support a mechanism of transcriptional repression whereby activated STAT5 functions downstream of IL-7 signalling to repress Bcl-6 expression. This finding is similar to the inverse correlation between IL-2-dependent STAT5 activation and Bcl-6 expression that we reported in a prior study12. Interestingly, STAT5 has been shown to interact with the histone methyltransferase, Ezh2, downstream of IL-7 signalling56. As such, future experiments that determine whether there are cytokine-dependent differences to the composition of the STAT5 complex that binds to the Bcl6 locus could provide insight into how IL-7 and IL-2 harbour non-redundant roles in the regulation of immune cell development and function, despite signalling through a common downstream transcriptional regulator, STAT5 (refs 54, 55).
Collectively, this study supports a model in which effector TH1 cells co-initiate TFH and TCM gene programs when IL-2 signals begin to wane. Our finding that antigen-specific effector CD4+ T cells upregulate the dual expression of IL-6Rα and IL-7R at late time points post influenza infection suggests that exposure to either cytokine could be a key determinant in further differentiation events and long-term cellular function. Although these findings may be indicative of divergence to either a TFH- or TCM-specific gene program, they do not preclude the possibility that plasticity exists between these cell states to influence trafficking between the T-cell and B-cell zones of secondary lymphoid tissues to provide a more comprehensive immune response. It is also possible that the simultaneous expression of IL-6Rα and IL-7R allow for precise modulation of Bcl-6 expression. A rheostatic model such as this may allow for modest Bcl-6 expression, sufficient to repress Blimp-1 expression and initiate the TCM transcriptional program, while also repressing the enhanced levels of Bcl-6 required for TFH development via IL-7 signalling. Future studies that address these possibilities by examining the functional properties of the IL-6Rα+IL-7R+ CD4+ cells described here will be critical to enhance our understanding of the relationship between the TFH and TCM cell types.
Methods
Primary cells and cell culture
Primary naive CD4+ T cells were isolated from the spleen and lymph nodes of sex- and age-matched (5–8-week old) wild-type C57BL/6 mice using the MagCellect kit (R&D, MAGM205), consistently providing a 90–95% pure population. Following isolation, cells were cultured on plate-bound αCD3/αCD28 in TH1 polarizing conditions (α-IL-4 (5 μg ml−1) and IL-12 (5 ng ml−1)). After 3 days, cells were removed from αCD3/αCD28 stimulation, split and cultured for an additional 2 days in either high (250 U ml−1) or low IL-2 (10 U ml−1) conditions to generate effector TH1 or TFH-like cells, respectively12. Low IL-2-treated cells were then treated with IL-6 (10 ng ml−1) or IL-7 (10 ng ml−1) unless otherwise indicated. Following a 24-h incubation, cells were collected for analysis. Primary T-cell transfections were performed with the 4D Lonza nucleofection system (program DN-100, solution P3). The Institutional Animal Care and Use Committees of Virginia Tech and the University of Alabama at Birmingham approved all the experimentation involving the use of mice. All the methods were performed in accordance with the approved guidelines.
Murine EL4 T cells (TIB-39, ATCC) were cultured in RPMI supplemented with 10% FBS and 1% Pen-strep. EL4 transfections were performed using the Lonza 4D nucleofection system (program CM-120, solution SF). Immunoblot analysis was used to assess the expression levels of all transfected proteins.
RNA purification and quantitative PCR with reverse transcription
Cells were collected on day 5, 6 or 7 (dependent on experimental design) and RNA was purified using the Machery Nagel RNA purification kit. Complementary DNA was prepared using the First Strand Superscript II Synthesis System (Invitrogen). Quantitative PCR with reverse transcription (qRT–PCR) reactions were performed with the cDNA and gene-specific primers (Supplementary Table 1) and SYBR green master mix (Bio-Rad). For the experiments in Figs 1 and 5a, PrimePCR custom plates (Bio-Rad) were used. All the samples were normalized to the Rps18 control with graphs representing data normalized to the indicated comparison condition.
siRNA experiments
For the siRNA experiments, both control and Prdm1-specific siRNAs were obtained from Dharmacon (D-001210-01-20, D-043069; sequences in Supplementary Table 2). Primary TH1 cells were transfected with the indicated siRNAs on day 5. Following nucleofection with siRNA, primary cells were allowed to recover in high IL-2 conditions for 24 h before gene expression analysis. Efficiency of knockdown was determined by qRT–PCR and immunoblot analysis for Prdm1 transcript and Blimp-1 protein expression, respectively.
Immunoblot analysis
An equal number of cells were collected and subjected to immunoblot analysis to determine protein expression levels of Bcl-6 (BD Biosciences, 561520, dilution 1:500), Blimp-1 (Genscript, A01647, dilution 1:500), STAT5 (Santa Cruz, sc-835, dilution 1:5,000), phospho-STAT5 (pY694, BD Biosciences, 611964, dilution 1:5,000) and V5-tagged proteins (Invitrogen, R960-25, dilution 1:5,000). In brief, separation of lysates by SDS–polyacrylamide gel electrophoresis was followed by immunoblot analysis. GAPDH (Santa Cruz, sc-25778, dilution 1:2,500) or β-actin (Genscript, A00730, dilution 1:10,000) expression was monitored to ensure equal protein loading. Additional information for antibodies can be found in Supplementary Table 3 and uncropped versions of all immunoblots are provided in Supplementary Fig. 5.
ChIP assay
The ChIP assay was performed as published12. In brief, chromatin was harvested from TFH/TCM-like cells treated with and without IL-7 as indicated. Chromatin was incubated with antibodies to either STAT5 (Santa Cruz, sc-835x, 5 μg per IP) or IgG (Abcam, ab6709, 5 μg per IP) control and the precipitated DNA was analysed by qPCR with gene-specific primers (Supplementary Table 1). Samples were normalized to a standardized total input DNA control followed by subtraction of the IgG antibody as a control for the nonspecific background. The final value represents the percent enrichment of STAT5-specific signal.
Promoter–reporter analysis
Sell (−1,755 to +79 bp), Ccr7 (−1,470 to +113 bp) and Tbx21 (−1,893 to +194 bp) promoter–reporter vectors were prepared by cloning regulatory regions of each gene into the pGL3-basic luciferase reporter construct (Promega). EL4 cells were co-transfected with the promoter–reporter constructs in combination with the indicated expression vectors as well as a TK-renilla control plasmid (Promega) to normalize for transfection efficiency. Transfections were harvested after 16–24 h and samples were analysed with the Dual-Luciferase Reporter system (Promega).
Flow cytometry
Fluorochrome-labelled α-IL-6Rα (P22272, dilution 1:10), α-IL-7R (A7R34, dilution 1:10), α-CD62L (95218, dilution 1:20), α-Cxcr5 (614641, dilution 1:10) and respective isotype control antibodies were purchased from R&D. α-Ccr7 (4B12, dilution 1:50), α-IL-2Rα (PC61.5, dilution 1:50), α-IL-7R (SB/199, dilution 1:10) and α-CD62L (MEL-14, dilution 1:100) were purchased from eBioscience. Non-viable cells were excluded using e520 or e450 viability dye (eBioscience), Sytox Green or Blue (Life Technologies) or propidium iodide (BD Biosciences). For the staining procedure, cells were collected on day 6 (3 days post transition to low IL-2 conditions) and subjected to labelling and sorting as indicated. Briefly, cells were pelleted, washed in 500 μl of 1 × FACS buffer (2% FBS, 1% BSA, 0.1% NaNH3), and then stained with indicated fluorochrome-conjugated antibody. Following staining with fluorochrome-conjugated antibodies and viability dye, cells were washed three times in 1 × FACS buffer and then resuspended for analysis on either an Accuri C6 flow cytometer or Sony SH800. All the obtained data were analysed using FlowJo Software.
Cell sorting and droplet digital PCR
Day 6 IL-6Rα+/IL-7R+ DP cells were sorted (∼500,000 cells) using a Sony SH800 using a 100 μm chip under purity-sort mode and cDNA was prepared as described above (see qRT–PCR). Droplet Digital PCR reactions were performed using gene-specific primers diluted in 2 × master mix (Qx200 ddPCR EvaGreen Supermix, Bio-Rad). Droplets were generated using a Bio-Rad Automated Droplet Generator and end-point PCR was performed. Droplets were then read using a Qx200 Digital PCR Reader using QuantaSoft Software (Bio-Rad). All the primers were diluted to optimize signal-to-noise and thresholds were set using non-template control wells. Absolute counts were normalized to Rps18 and then fold change was determined.
Influenza virus infections and in vivo analysis
Influenza virus infections were performed intranasally with 6,500 VFU of A/PR8/34 (PR8) in 100 μl of PBS. Cell suspensions from mLNs were prepared by passing tissues through nylon mesh. Cells from mLNs were resuspended in 150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM EDTA for 5 min to lyse red cells. Cell suspensions were then filtered through a 70 μm nylon cell strainer (BD Biosciences), washed and resuspended in PBS with 5% donor calf serum and 10 μg ml−1 FcBlock (2.4G2 -BioXCell) for 10 min on ice before staining with fluorochrome-conjugated antibodies or tetramer reagents. Fluorochrome-labelled α-PD-1 (J43, dilution 1:100), α-IL-7R (A7R34, dilution 1:100) and α-IL-6Rα (D7725A7, dilution 1:100) were from eBioscience. Fluorochrome-labelled α-Bcl-6 (K112.91, dilution 1:50), α-Cxcr5 (2G-8, dilution 1:50) and α-CD4 (RM4-5, dilution 1:200) were from BD Biosciences. The IAbNP311-325 MHC class II tetramer was obtained from the NIH Tetramer Core Facility and used at a 1:100 dilution. Intracellular staining for Bcl-6 was performed using the mouse regulatory T-cell staining kit (eBioscience) following the manufacturer’s instructions. Flow cytometry was performed using a FACSCanto II (BD Biosciences) and analysed in FlowJo.
Statistics
All the data represent at least three independent experiments with the number of biological replicates indicated in the figure legend. Error bars represent the s.e.m. or s.d. as indicated. For statistical analysis, an unpaired t test was performed using GraphPad Prism online software. P values <0.05 were considered statistically significant.
Additional information
How to cite this article: McDonald, P. W. et al. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat. Commun. 7:10285 doi: 10.1038/ncomms10285 (2016).
References
Gasper, D. J., Tejera, M. M. & Suresh, M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 34, 121–146 (2014) .
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8(+) T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012) .
Strutt, T. M. et al. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 255, 149–164 (2013) .
Crotty, S. Follicular helper CD4 T cells (T(FH). Annu. Rev. Immunol. 29, 621–663 (2011) .
Ma, C. S., Deenick, E. K., Batten, M. & Tangye, S. G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 209, 1241–1253 (2012) .
Tangye, S. G., Ma, C. S., Brink, R. & Deenick, E. K. The good, the bad and the ugly—TFH cells in human health and disease. Nat. Rev. Immunol. 13, 412–426 (2013) .
Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014) .
Moon, H. et al. Early development in the peritoneal cavity of CD49dhigh Th1 memory phenotype CD4+ T cells with enhanced B cell helper activity. J. Immunol. 195, 564–575 (2015) .
Choi, Y. S. et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 190, 4014–4026 (2013) .
Lu, K. T. et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 35, 622–632 (2011) .
Nakayamada, S. et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931 (2011) .
Oestreich, K. J., Mohn, S. E. & Weinmann, A. S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 13, 405–411 (2012) .
Liu, X. et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 209, 1841–1852 (2012) .
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009) .
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009) .
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009) .
Crotty, S., Johnston, R. J. & Schoenberger, S. P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11, 114–120 (2010) .
Ichii, H. et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3, 558–563 (2002) .
Pepper, M., Pagan, A. J., Igyarto, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011) .
Ichii, H. et al. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int. Immunol. 19, 427–433 (2007) .
Kallies, A. Distinct regulation of effector and memory T-cell differentiation. Immunol. Cell. Biol. 86, 325–332 (2008) .
Kallies, A., Xin, A., Belz, G. T. & Nutt, S. L. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 31, 283–295 (2009) .
Rutishauser, R. L. et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009) .
Zhu, J. & Paul, W. E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238, 247–262 (2010) .
Ballesteros-Tato, A. et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856 (2012) .
Johnston, R. J., Choi, Y. S., Diamond, J. A., Yang, J. A. & Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 209, 243–250 (2012) .
Nurieva, R. I. et al. STAT5 negatively regulates T follicular helper (Tfh) cell generation and function. J. Biol. Chem. 287, 11234–11239 (2012) .
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010) .
Stelekati, E. et al. Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity 40, 801–813 (2014) .
Hale, J. S. et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 (2013) .
MacLeod, M. K. et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 186, 2889–2896 (2011) .
Weber, J. P., Fuhrmann, F. & Hutloff, A. T-follicular helper cells survive as long-term memory cells. Eur. J. Immunol. 42, 1981–1988 (2012) .
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007) .
Choi, Y. S. et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16, 980–990 (2015) .
Jeannet, G. et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl Acad. Sci. USA 107, 9777–9782 (2010) .
Wu, T. et al. TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep. 12, 2099–2110 (2015) .
Xu, L. et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat. Immunol. 16, 991–999 (2015) .
Zhou, X. et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33, 229–240 (2010) .
Oestreich, K. J. et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15, 957–964 (2014) .
Liao, W., Lin, J. X., Wang, L., Li, P. & Leonard, W. J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 12, 551–559 (2011) .
Li, J., Huston, G. & Swain, S. L. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198, 1807–1815 (2003) .
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008) .
Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013) .
Eto, D. et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 6, e17739 (2011) .
Poholek, A. C. et al. In vivo regulation of Bcl6 and T follicular helper cell development. J. Immunol. 185, 313–326 (2010) .
Carrette, F. & Surh, C. D. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin. Immunol. 24, 209–217 (2012) .
Cui, G. et al. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161, 750–761 (2015) .
Kim, K. et al. Characterization of an interleukin-7-dependent thymic cell line derived from a p53(-/-) mouse. J. Immunol. Methods 274, 177–184 (2003) .
Kitano, M. et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 34, 961–972 (2011) .
Hale, J. S. & Ahmed, R. Memory T follicular helper CD4 T cells. Front. Immunol. 6, 16 (2015) .
Basso, K. & Dalla-Favera, R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv. Immunol. 105, 193–210 (2010) .
Phan, R. T., Saito, M., Basso, K., Niu, H. & Dalla-Favera, R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat. Immunol. 6, 1054–1060 (2005) .
Martins, G. & Calame, K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu. Rev. Immunol. 26, 133–169 (2008) .
Liao, W., Lin, J. X. & Leonard, W. J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 23, 598–604 (2011) .
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009) .
Mandal, M. et al. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat. Immunol. 12, 1212–1220 (2011) .
Choi, Y. S., Yang, J. A. & Crotty, S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr. Opin. Immunol. 25, 366–372 (2013) .
Phan, R. T. & Dalla-Favera, R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 432, 635–639 (2004) .
Ranuncolo, S. M. et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat. Immunol. 8, 705–714 (2007) .
Lee, J. Y. et al. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity 42, 252–264 (2015) .
Weber, J. P. et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J. Exp. Med. 212, 217–233 (2015) .
Acknowledgements
This work was supported by start-up funds from VTCRI and an intramural research grant from the Virginia-Maryland College of Veterinary Medicine (to K.J.O.). Support for A.B.-T. was provided by the University of Alabama at Birmingham and the National Institutes of Health (R01 AI110480). We thank Dr. Irving Allen and Dr. Ansar Ahmed, as well as the faculty of the Virginia Tech Carilion Research Institute (VTCRI), for helpful discussions.
Author information
Authors and Affiliations
Contributions
P.W.M. assisted with the design of the study, performed experiments, analysed the data and wrote the manuscript. K.A.R., C.E.B., A.E.A. and M.D.P. performed the experiments and analysed the data. A.B.-T. designed, supervised and analysed all the experiments involving influenza infection. K.J.O. supervised the research, designed the study, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-5 and Supplementary Tables 1-3 (PDF 955 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
McDonald, P., Read, K., Baker, C. et al. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat Commun 7, 10285 (2016). https://doi.org/10.1038/ncomms10285
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms10285
This article is cited by
-
IL6 suppresses vaccine responses in neonates by enhancing IL2 activity on T follicular helper cells
npj Vaccines (2023)
-
Aiolos represses CD4+ T cell cytotoxic programming via reciprocal regulation of TFH transcription factors and IL-2 sensitivity
Nature Communications (2023)
-
Mucosal T follicular helper cells in SIV-infected rhesus macaques: contributing role of IL-27
Mucosal Immunology (2019)
-
Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose
Scientific Reports (2018)
-
Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection
Nature Immunology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.