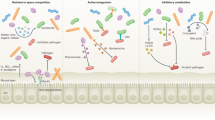
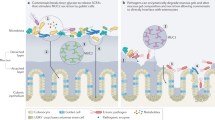
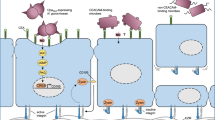
Abstract
The gut mucosa acts as a barrier against microbial invaders, whereas resident commensal and foreign invading bacteria interact intimately with the gut epithelium and influence the host cellular and immune systems. The epithelial barrier serves as an infectious foothold for many bacterial pathogens and as an entry port for pathogens to disseminate into deeper tissues. Enteric bacterial pathogens can efficiently infect the gut mucosa using highly sophisticated virulence mechanisms that allow bacteria to circumvent the defense barriers in the gut. We provide an overview of the components of the mucosal barrier and discuss the bacterial stratagems that circumvent these barriers with particular emphasis on the roles of bacterial effector proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim, M. et al. Bacterial interactions with the host epithelium. Cell Host Microbe 8, 20–35 (2010).
Galán, J.E. & Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573 (2006).
Marteyn, B. et al. Modulation of Shigella virulence in response to available oxygen in vivo Nature 465, 355–358 (2010).
Arpaia, N. et al. TLR signaling is required for Salmonella typhimurium virulence. Cell 144, 675–688 (2011).
Yu, X.J., McGourty, K., Liu, M., Unsworth, K.E. & Holden, D.W. pH sensing by intracellular Salmonella induces effector translocation. Science 328, 1040–1043 (2010).
McGhie, E.J., Brawn, L.C., Hume, P.J., Humphreys, D. & Koronakis, V. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12, 117–124 (2009).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 119–129 (2007).
Stecher, B. & Hardt, W.D. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 14, 82–91 (2011).
Keeney, K.M. & Finlay, B.B. Enteric pathogen exploitation of the microbiota-generated nutrient environment of the gut. Curr. Opin. Microbiol. 14, 92–98 (2011).
Savage, D.C., Siegel, J.E., Snellen, J.E. & Whitt, D.D. Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Appl. Environ. Microbiol. 42, 996–1001 (1981).
Chowdhury, S.R. et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics 8, 215 (2007).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011). This study shows that acetate produced by Bifidobacteria species prevents lethal infection and epithelial cell death induced by EHEC O157.
Maslowski, K.M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Guilloteau, P. et al. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23, 366–384 (2010).
Raqib, R. et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. USA 103, 9178–9183 (2006).
Hooper, L.V. & Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). This study reports that Clostridium species induce regulatory T cells and maintain immunological homeostasis via stimulating matrix metalloprotease-TGF-β signaling.
Willing, B.P., Russell, S.L. & Finlay, B.B. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Garner, C.D. et al. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect. Immun. 77, 2691–2702 (2009).
Huang, Y., Suyemoto, M., Garner, C.D., Cicconi, K.M. & Altier, C. Formate acts as a diffusible signal to induce Salmonella invasion. J. Bacteriol. 190, 4233–4241 (2008).
Stecher, B. et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5, 2177–2189 (2007). This report shows the benefit of intestinal inflammation in promoting the colonization of bacterial pathogens. The authors showed that S. Typhimurium-induced host inflammation changes the composition and suppresses the growth of the microbiota, thereby overcoming colonization resistance.
Lawley, T.D. et al. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 76, 403–416 (2008).
Winter, S.E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella Nature 467, 426–429 (2010). This study, in addition to ref. 23, shows the benefits of intestinal inflammation during S. Typhimurium infection. S. Typhimurium use tetrathionate, which is produced as a result of inflammation, as an electron acceptor and gains a growth advantage to overcome the host microbiota.
McGuckin, M.A., Lindén, S.K., Sutton, P. & Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011). This review explores the role of mucin as a barrier to bacterial infection. It also describes the interaction between bacterial pathogens and the mucus layer.
Dharmani, P., Srivastava, V., Kissoon-Singh, V. & Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 1, 123–135 (2009).
Li, J.D. et al. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 95, 5718–5723 (1998).
Lemjabbar, H. & Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8, 41–46 (2002).
McAuley, J.L. et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324 (2007).
McGuckin, M.A. et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210–1218 (2007).
Lindén, S.K. et al. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 5, e1000617 (2009). This study shows the importance of MUC1 in limiting H. pylori colonization. The authors found that mucin acts as decoy that is released from the epithelial surface in response to bacterial binding, thereby preventing prolonged infection.
Tu, Q.V., McGuckin, M.A. & Mendz, G.L. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J. Med. Microbiol. 57, 795–802 (2008).
Ramos, H.C., Rumbo, M. & Sirard, J.C. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12, 509–517 (2004).
Henderson, I.R., Czeczulin, J., Eslava, C., Noriega, F. & Nataro, J.P. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli Infect. Immun. 67, 5587–5596 (1999).
Grys, T.E., Siegel, M.B., Lathem, W.W. & Welch, R.A. The StcE protease contributes to intimate adherence of enterohemorrhagic Escherichia coli O157:H7 to host cells. Infect. Immun. 73, 1295–1303 (2005).
Silva, A.J., Pham, K. & Benitez, J.A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae Microbiology 149, 1883–1891 (2003).
Szabady, R.L., Yanta, J.H., Halladin, D.K., Schofield, M.J. & Welch, R.A. TagA is a secreted protease of Vibrio cholerae that specifically cleaves mucin glycoproteins. Microbiology 157, 516–525 (2011).
Mantle, M. & Rombough, C. Growth in and breakdown of purified rabbit small intestinal mucin by Yersinia enterocolitica Infect. Immun. 61, 4131–4138 (1993).
Gumbiner, B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357 (1996).
Popoff, M.R. & Geny, B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim. Biophys. Acta 1788, 797–812 (2009).
Wang, F. et al. Interferon-g and tumor necrosis factor-a synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409–419 (2005).
Graham, W.V. et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J. Biol. Chem. 281, 26205–26215 (2006).
Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 180, 5653–5661 (2008).
Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Guttman, J.A. & Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841 (2009). This review highlights the role of tight junctions as a component of the epithelial barrier and describes how bacterial pathogens target and alter tight junctions during infection.
Croxen, M.A. & Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 (2010).
Alto, N.M. et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124, 133–145 (2006).
Arbeloa, A. et al. Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens. Cell. Microbiol. 10, 1429–1441 (2008).
Simovitch, M. et al. EspM inhibits pedestal formation by enterohaemorrhagic Escherichia coli and enteropathogenic E. coli and disrupts the architecture of a polarized epithelial monolayer. Cell. Microbiol. 12, 489–505 (2010).
Arbeloa, A. et al. EspM2 is a RhoA guanine nucleotide exchange factor. Cell. Microbiol. 12, 654–664 (2010).
Thanabalasuriar, A. et al. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli Cell. Microbiol. 12, 31–41 (2010).
Flynn, A.N. & Buret, A.G. Tight junctional disruption and apoptosis in an in vitro model of Citrobacter rodentium infection. Microb. Pathog. 45, 98–104 (2008).
Babbin, B.A., Sasaki, M., Gerner-Schmidt, K.W., Nusrat, A. & Klapproth, J.M. The bacterial virulence factor lymphostatin compromises intestinal epithelial barrier function by modulating rho GTPases. Am. J. Pathol. 174, 1347–1357 (2009).
Casselli, T., Lynch, T., Southward, C.M., Jones, B.W. & DeVinney, R. Vibrio parahaemolyticus inhibition of Rho family GTPase activation requires a functional chromosome I type III secretion system. Infect. Immun. 76, 2202–2211 (2008).
Yarbrough, M.L. et al. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323, 269–272 (2009). This study shows that VopS has AMPylation activity and modifies conserved threonine residues in Rho GTPase, thereby disrupting Rho GTPase signaling and regulating actin cytoskeleton remodeling.
Boyle, E.C., Brown, N.F. & Finlay, B.B. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell. Microbiol. 8, 1946–1957 (2006).
Bruno, V.M. et al. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5, e1000538 (2009).
Müller, A.J.D. et al. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe 20, 125–136 (2009).
Fischer, W., Prassl, S. & Haas, R. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori Curr. Top. Microbiol. Immunol. 337, 129–171 (2009).
Amieva, M.R. et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434 (2003).
Bagnoli, F., Buti, L., Tompkins, L., Covacci, A. & Amieva, M.R. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. USA 102, 16339–16344 (2005).
Saadat, I. et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333 (2007).
Papini, E. et al. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Invest. 102, 813–820 (1998).
Wroblewski, L.E. et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136, 236–246 (2009).
Lapointe, T.K., O'Connor, P.M., Jones, N.L., Menard, D. & Buret, A.G. Interleukin-1 receptor phosphorylation activates Rho kinase to disrupt human gastric tight junctional claudin-4 during Helicobacter pylori infection. Cell. Microbiol. 12, 692–703 (2010).
Madara, J.L. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am. J. Pathol. 137, 1273–1281 (1990).
Watson, A.J., Duckworth, C.A., Guan, Y. & Montrose, M.H. Mechanisms of epithelial cell shedding in the Mammalian intestine and maintenance of barrier function. Ann. NY Acad. Sci. 1165, 135–142 (2009).
Piguet, P.F., Vesin, C., Donati, Y. & Barazzone, C. TNF-induced enterocyte apoptosis and detachment in mice: induction of caspases and prevention by a caspase inhibitor, ZVAD-fmk. Lab. Invest. 79, 495–500 (1999).
Marchiando, A.M. et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140, 1208–1218 (2011).
Ashida, H. et al. Cell death and infection: a double-edged sword for host and pathogen survival. J. Cell Biol. (in the press).
Carneiro, L.A. et al. Shigella induces mitochondrial dysfunction and cell death in nonmyeloid cells. Cell Host Microbe 5, 123–136 (2009).
Paesold, G., Guiney, D.G., Eckmann, L. & Kagnoff, M.F. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell. Microbiol. 4, 771–781 (2002).
Schauser, K. & Larsson, L.I. Programmed cell death and cell extrusion in rat duodenum: a study of expression and activation of caspase-3 in relation to C-jun phosphorylation, DNA fragmentation and apoptotic morphology. Histochem. Cell Biol. 124, 237–243 (2005).
Jones, R.M. et al. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe 3, 233–244 (2008).
Du, F. & Galán, J.E. Selective inhibition of type III secretion activated signaling by the Salmonella effector AvrA. PLoS Pathog. 5, e1000595 (2009).
Knodler, L.A., Finlay, B.B. & Steele-Mortimer, O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280, 9058–9064 (2005).
Kum, W.W., Lo, B.C., Yu, H.B. & Finlay, B.B. Protective role of Akt2 in Salmonella enterica serovar typhimurium-induced gastroenterocolitis. Infect. Immun. 79, 2554–2566 (2011).
Nougayrède, J.P. & Donnenberg, M.S. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6, 1097–1111 (2004).
Nagai, T., Abe, A. & Sasakawa, C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280, 2998–3011 (2005).
Nougayrède, J.P., Foster, G.H. & Donnenberg, M.S. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell. Microbiol. 9, 680–693 (2007).
Ki, M.R. et al. Differential regulation of ERK1/2 and p38 MAP kinases in VacA-induced apoptosis of gastric epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G635–G647 (2008).
Amcheslavsky, A., Jiang, J. & Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila Cell Stem Cell 4, 49–61 (2009).
Pitsouli, C., Apidianakis, Y. & Perrimon, N. Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe 6, 301–307 (2009).
Sellin, J.H., Wang, Y., Singh, P. & Umar, S. β-Catenin stabilization imparts crypt progenitor phenotype to hyperproliferating colonic epithelia. Exp. Cell Res. 315, 97–109 (2009).
Mimuro, H. et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2, 250–263 (2007).
Wessler, S. & Backert, S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori Trends Microbiol. 16, 397–405 (2008).
Chang, Y.J. et al. Mechanisms for Helicobacter pylori CagA-induced cyclin D1 expression that affect cell cycle. Cell. Microbiol. 8, 1740–1752 (2006).
Iwai, H. et al. A bacterial effector targets Mad2L2, an APC inhibitor, to modulate host cell cycling. Cell 130, 611–623 (2007).
Cui, J. et al. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 329, 1215–1218 (2010). This study reveals that Cif deaminates NEDD8 and interferes with its function, resulting in cell cycle arrest.
Jubelin, G. et al. Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways. PLoS Pathog. 6, e1001128 (2010).
Morikawa, H. et al. The bacterial effector Cif interferes with SCF ubiquitin ligase function by inhibiting deneddylation of Cullin1. Biochem. Biophys. Res. Commun. 401, 268–274 (2010).
Samba-Louaka, A. et al. Bacterial cyclomodulin Cif blocks the host cell cycle by stabilizing the cyclin-dependent kinase inhibitors p21 and p27. Cell. Microbiol. 10, 2496–2508 (2008).
Yao, Q. et al. A bacterial type III effector family uses the papain-like hydrolytic activity to arrest the host cell cycle. Proc. Natl. Acad. Sci. USA 106, 3716–3721 (2009).
Hemrajani, C. et al. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc. Natl. Acad. Sci. USA 107, 3129–3134 (2010).
Levy, S.B. & Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10, S122–S129 (2004).
Marra, A. Targeting virulence for antibacterial chemotherapy: identifying and characterising virulence factors for lead discovery. Drugs R D. 7, 1–16 (2006).
Nordfelth, R., Kauppi, A.M., Norberg, H.A., Wolf-Watz, H. & Elofsson, M. Small-molecule inhibitors specifically targeting type III secretion. Infect. Immun. 73, 3104–3114 (2005).
Negrea, A. et al. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 51, 2867–2876 (2007).
Veenendaal, A.K., Sundin, C. & Blocker, A.J. Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J. Bacteriol. 191, 563–570 (2009).
Acknowledgements
This work was supported by Grant-in-Aid for Specially Promoted Research (23000012 to C.S.); a Grant-in-Aid for Scientific Research (S) (20229006 to C.S.); a Grant-in-Aid for Young Scientists (A) (23689027 to M.K.); a Grant-in-Aid for Young Scientists (B) (23790471 to M.O. and 23790472 to H.A.), a Grant-in-Aid for Scientific Research (B) (23390102 to H.M.); a Grant-in-Aid for Challenging Exploratory Research (23659220 to H.M.); and a grant from the Japan Initiative for Global Research Network on Infectious Diseases to C.S. from the Ministry of Education, Culture, Sports, Science and Technology. Part of this work was supported by grants from the Naito Foundation (to H.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ashida, H., Ogawa, M., Kim, M. et al. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol 8, 36–45 (2012). https://doi.org/10.1038/nchembio.741
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.741
This article is cited by
-
Cleavage of cell junction proteins as a host invasion strategy in leptospirosis
Applied Microbiology and Biotechnology (2024)
-
Infiltration to infection: key virulence players of Helicobacter pylori pathogenicity
Infection (2024)
-
Phytogenic feed additives alleviate pathogenic Escherichia coli-induced intestinal damage through improving barrier integrity and inhibiting inflammation in weaned pigs
Journal of Animal Science and Biotechnology (2022)
-
The deubiquitinase OTUD1 inhibits colonic inflammation by suppressing RIPK1-mediated NF-κB signaling
Cellular & Molecular Immunology (2022)
-
Functional implications of the CpG island methylation in the pathogenesis of celiac disease
Molecular Biology Reports (2022)