Abstract
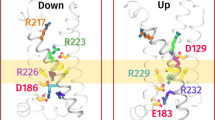
Allostery provides a critical control over enzyme activity, biasing the catalytic site between inactive and active states. We found that the Ciona intestinalis voltage-sensing phosphatase (Ci-VSP), which modifies phosphoinositide signaling lipids (PIPs), has not one but two sequential active states with distinct substrate specificities, whose occupancy is allosterically controlled by sequential conformations of the voltage-sensing domain (VSD). Using fast fluorescence resonance energy transfer (FRET) reporters of PIPs to monitor enzyme activity and voltage-clamp fluorometry to monitor conformational changes in the VSD, we found that Ci-VSP switches from inactive to a PIP3-preferring active state when the VSD undergoes an initial voltage-sensing motion and then into a second PIP2-preferring active state when the VSD activates fully. This two-step allosteric control over a dual-specificity enzyme enables voltage to shape PIP concentrations in time, and provides a mechanism for the complex modulation of PIP-regulated ion channels, transporters, cell motility, endocytosis and exocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Changeux, J.P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb. Symp. Quant. Biol. 26, 313–318 (1961).
Monod, J. & Jacob, F. General conclusions—teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb. Symp. Quant. Biol. 26, 389–401 (1961).
Monod, J., Wyman, J. & Changeux, J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Hilser, V.J. An ensemble view of allostery. Science 327, 653–654 (2010).
Motlagh, H.N., Wrabl, J.O., Li, J. & Hilser, V.J. The ensemble nature of allostery. Nature 508, 331–339 (2014).
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005).
Suh, B.C. & Hille, B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195 (2008).
Worby, C.A. & Dixon, J.E. Phosphoinositide phosphatases: emerging roles as voltage sensors? Mol. Interv. 5, 274–277 (2005).
Hobiger, K., Utesch, T., Mroginski, M.A. & Friedrich, T. Coupling of Ci-VSP modules requires a combination of structure and electrostatics within the linker. Biophys. J. 102, 1313–1322 (2012).
Hobiger, K., Utesch, T., Mroginski, M.A., Seebohm, G. & Friedrich, T. The linker pivot in Ci-VSP: the key to unlock catalysis. PLoS ONE 8, e70272 (2013).
Hossain, M.I. et al. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J. Biol. Chem. 283, 18248–18259 (2008).
Kohout, S.C. et al. Electrochemical coupling in the voltage-dependent phosphatase Ci-VSP. Nat. Chem. Biol. 6, 369–375 (2010).
Kohout, S.C., Ulbrich, M.H., Bell, S.C. & Isacoff, E.Y. Subunit organization and functional transitions in Ci-VSP. Nat. Struct. Mol. Biol. 15, 106–108 (2008).
Murata, Y. & Okamura, Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J. Physiol. (Lond.) 583, 875–889 (2007).
Villalba-Galea, C.A., Miceli, F., Taglialatela, M. & Bezanilla, F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J. Gen. Physiol. 134, 5–14 (2009).
Hobiger, K. & Friedrich, T. Voltage sensitive phosphatases: emerging kinship to protein tyrosine phosphatases from structure-function research. Front. Pharmacol. 6, 20 (2015).
Kalli, A.C., Devaney, I. & Sansom, M.S. Interactions of phosphatase and tensin homologue (PTEN) proteins with phosphatidylinositol phosphates: insights from molecular dynamics simulations of PTEN and voltage sensitive phosphatase. Biochemistry 53, 1724–1732 (2014).
Lacroix, J. et al. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J. Biol. Chem. 286, 17945–17953 (2011).
Okamura, Y. & Dixon, J.E. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology (Bethesda) 26, 6–13 (2011).
Halaszovich, C.R., Schreiber, D.N. & Oliver, D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J. Biol. Chem. 284, 2106–2113 (2009).
Iwasaki, H. et al. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 105, 7970–7975 (2008).
Kurokawa, T. et al. 3′ Phosphatase activity toward phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] by voltage-sensing phosphatase (VSP). Proc. Natl. Acad. Sci. USA 109, 10089–10094 (2012).
Liu, L. et al. A glutamate switch controls voltage-sensitive phosphatase function. Nat. Struct. Mol. Biol. 19, 633–641 (2012).
Matsuda, M. et al. Crystal structure of the cytoplasmic phosphatase and tensin homolog (PTEN)-like region of Ciona intestinalis voltage-sensing phosphatase provides insight into substrate specificity and redox regulation of the phosphoinositide phosphatase activity. J. Biol. Chem. 286, 23368–23377 (2011).
Baker, O.S., Larsson, H.P., Mannuzzu, L.M. & Isacoff, E.Y. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in shaker K+ channel gating. Neuron 20, 1283–1294 (1998).
Pathak, M., Kurtz, L., Tombola, F. & Isacoff, E. The cooperative voltage sensor motion that gates a potassium channel. J. Gen. Physiol. 125, 57–69 (2005).
Schoppa, N.E. & Sigworth, F.J. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111, 313–342 (1998).
Seoh, S.A., Sigg, D., Papazian, D.M. & Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167 (1996).
Villalba-Galea, C.A., Sandtner, W., Starace, D.M. & Bezanilla, F. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA 105, 17600–17607 (2008).
Sakata, S. & Okamura, Y. Phosphatase activity of the voltage-sensing phosphatase, VSP, shows graded dependence on the extent of activation of the voltage sensor. J. Physiol. (Lond.) 592, 899–914 (2014).
Idevall-Hagren, O. & De Camilli, P. Detection and manipulation of phosphoinositides. Biochim. Biophys. Acta 1851, 736–745 (2015).
Mavrantoni, A., Thallmair, V., Leitner, M.G., Schreiber, D.N., Oliver, D. & Halaszovich, C.R. A method to control phosphoinositides and to analyze PTEN function in living cells using voltage sensitive phosphatases. Front. Pharmacol. 6, 68 (2015).
Sakata, S., Hossain, M.I. & Okamura, Y. Coupling of the phosphatase activity of Ci-VSP to its voltage sensor activity over the entire range of voltage sensitivity. J. Physiol. (Lond.) 589, 2687–2705 (2011).
Sato, M., Ueda, Y., Takagi, T. & Umezawa, Y. Production of PtdInsP(3) at endomembranes is triggered by receptor endocytosis. Nat. Cell Biol. 5, 1016–1022 (2003).
Li, Q. et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21, 244–252 (2014).
Nishioka, T. et al. Rapid turnover rate of phosphoinositides at the front of migrating MDCK cells. Mol. Biol. Cell 19, 4213–4223 (2008).
Yoshizaki, H., Mochizuki, N., Gotoh, Y. & Matsuda, M. Akt-PDK1 complex mediates epidermal growth factor-induced membrane protrusion through Ral activation. Mol. Biol. Cell 18, 119–128 (2007).
Cha, A. & Bezanilla, F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 19, 1127–1140 (1997).
Gandhi, C.S., Loots, E. & Isacoff, E.Y. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron 27, 585–595 (2000).
Koch, H.P. et al. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 105, 9111–9116 (2008).
Mannuzzu, L.M., Moronne, M.M. & Isacoff, E.Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271, 213–216 (1996).
Pathak, M.M. et al. Closing in on the resting state of the Shaker K(+) channel. Neuron 56, 124–140 (2007).
Tombola, F., Pathak, M.M. & Isacoff, E.Y. How far will you go to sense voltage? Neuron 48, 719–725 (2005).
Castle, P.M., Zolman, K.D. & Kohout, S.C. Voltage-sensing phosphatase modulation by a C2 domain. Front. Pharmacol. 6, 63 (2015).
Catterall, W.A. Molecular-properties of voltage-sensitive sodium-Channels. Annu. Rev. Biochem. 55, 953–985 (1986).
Guy, H.R. & Seetharamulu, P. Molecular-model of the action-potential sodium-channel. Proc. Natl. Acad. Sci. USA 83, 508–512 (1986).
Papazian, D.M. et al. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron 14, 1293–1301 (1995).
Papazian, D.M., Timpe, L.C., Jan, Y.N. & Jan, L.Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature 349, 305–310 (1991).
Tombola, F., Pathak, M.M. & Isacoff, E.Y. How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 22, 23–52 (2006).
Campos, F.V., Chanda, B., Roux, B. & Bezanilla, F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. USA 104, 7904–7909 (2007).
Chamberlin, A. et al. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 111, E273–E282 (2014).
Lacroix, J.J. & Bezanilla, F. Control of a final gating charge transition by a hydrophobic residue in the S2 segment of a K+ channel voltage sensor. Proc. Natl. Acad. Sci. USA 108, 6444–6449 (2011).
Lacroix, J.J., Hyde, H.C., Campos, F.V. & Bezanilla, F. Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl. Acad. Sci. USA 111, E1950–E1959 (2014).
Pless, S.A., Galpin, J.D., Niciforovic, A.P. & Ahern, C.A. Contributions of counter-charge in a potassium channel voltage-sensor domain. Nat. Chem. Biol. 7, 617–623 (2011).
Starace, D.M. & Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553 (2004).
Lacroix, J.J. et al. Intermediate state trapping of a voltage sensor. J. Gen. Physiol. 140, 635–652 (2012).
Perozo, E., Santacruz-Toloza, L., Stefani, E., Bezanilla, F. & Papazian, D.M. S4 mutations alter gating currents of Shaker K channels. Biophys. J. 66, 345–354 (1994).
Tao, X., Lee, A., Limapichat, W., Dougherty, D.A. & MacKinnon, R. A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010).
Villalba-Galea, C.A., Frezza, L., Sandtner, W. & Bezanilla, F. Sensing charges of the Ciona intestinalis voltage-sensing phosphatase. J. Gen. Physiol. 142, 543–555 (2013).
Marchler-Bauer, A. et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229 (2011).
Acknowledgements
We thank Y. Okamura (Osaka University) for Ci-VSP, M. Matsuda (Kyoto University) for Fllip-pm, T. Meyer (Stanford University) for GFP-PLC-PH, T. Balla (US National Institutes of Health) for GFP-TAPP-PH, H. Okada, C. Stanley and Z. Fu for technical support, as well as A. Reiner, S. Bharill, S. Kohout, E. Carroll and other current and former members of the Isacoff laboratory for guidance on analysis and helpful discussions. This work was supported by the US National Institutes of Health (R01GM117051 and T32GM008295; E.Y.I.) as well as fellowship support for provided by the UC Berkeley Chancellors Fellowship for Graduate Study (S.S.G.).
Author information
Authors and Affiliations
Contributions
E.Y.I. and S.S.G. conceived the study, analyzed the data, directed the evolution of the project and wrote the paper. S.S.G. conducted the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–8. (PDF 1065 kb)
Rights and permissions
About this article
Cite this article
Grimm, S., Isacoff, E. Allosteric substrate switching in a voltage-sensing lipid phosphatase. Nat Chem Biol 12, 261–267 (2016). https://doi.org/10.1038/nchembio.2022
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2022
This article is cited by
-
Critical contributions of pre-S1 shoulder and distal TRP box in DAG-activated TRPC6 channel by PIP2 regulation
Scientific Reports (2022)
-
A126 in the active site and TI167/168 in the TI loop are essential determinants of the substrate specificity of PTEN
Cellular and Molecular Life Sciences (2018)