Abstract
Mast cells (MCs) are major effector cells contributing to allergic conditions. When activated, they can release large amounts of active proteases, including chymase from their secretory granules. Here we assessed the role of the chymase mouse mast cell protease 4 (mMCP-4) in allergic airway inflammation induced by house-dust mite (HDM) extract. mMCP-4−/− mice demonstrated elevated airway reactivity and eosinophilia compared with wild-type (WT) animals, suggesting a protective role for mMCP-4 during the late inflammatory phase of the disease. However, mMCP-4 also contributed to the sensitization phase, as indicated by higher levels of serum immunoglobulin E in mMCP-4−/− vs. WT mice and higher levels of cytokines secreted by HDM-restimulated mMCP-4−/− vs. WT splenocytes. In line with a contribution of mMCP-4 in the early stages of disease, HDM extract directly induced chymase secretion from MCs. The elevated airway and inflammatory responses of mMCP-4−/− mice were associated with a profound increase in the levels of interleukin (IL)-33 in the lung tissue. Moreover, WT MCs degraded IL-33 more efficiently than did MCs lacking mMCP-4. Together, our findings identify a protective role of a MC chymase in a physiologically relevant model for airway inflammation and suggest that chymase-mediated regulation of IL-33 can account for this protective function.
Similar content being viewed by others
Introduction
Mast cells (MCs) are key effector cells in allergic conditions such as asthma but they are also regarded as regulatory cells, fine-tuning immune responses in various ways.1 When MCs are activated, they may degranulate and thereby release a number of preformed compounds from their secretory granules, including bioactive amines, cytokines, proteoglycans, and various MC-specific proteases, the latter encompassing chymases, tryptases, and carboxypeptidase A3.2, 3 Activated MCs may also respond by de novo synthesis of additional pro-inflammatory compounds.4
The MC proteases constitute a major part of the total content of the MC granules. Hence, large amounts of proteases are released upon MC degranulation, and it is likely that they will have a major impact on any condition in which MC degranulation occurs.3 Out of the various murine chymases, mouse mast cell protease 4 (mMCP-4) is considered to be the functional counterpart to the single human chymase.3, 5 In a recent study, we showed that mMCP-4 has protective properties in an acute model of allergic airway inflammation induced by intraperitoneal sensitization followed by intranasal challenge with ovalbumin (OVA).6 However, the exact mechanism by which mMCP-4 dampens airway responses is not known. Moreover, it is important to emphasize that the OVA model has been questioned in terms of relevance for human asthma.7, 8 The aim of this investigation was therefore to investigate the role of chymase in a physiologically relevant model for asthma, and also to address the mechanism by which chymase influences airway responses.
House-dust mite (HDM) is a prevalent allergen of humans and is a major cause of human asthma.9 HDM-induced allergic airway inflammation in mice shares many of the features of human asthma, including upregulated T helper type 2 (Th2) cytokines and immunoglobulin E (IgE) synthesis, lung eosinophilia, and airway hyperresponsiveness and is therefore emerging as a physiologically relevant model for human asthma.10, 11
Here we evaluated the impact of mMCP-4 on HDM-induced airway responses. We show that the absence of mMCP-4 leads to markedly elevated lung eosinophilia, IgE responses, airway smooth muscle (ASM) thickening, and elevated airway reactivity at high doses of metacholine, indicating a protective role for MC chymase. Further, our data suggest that the protective function of chymase mechanistically can be linked to effects on interleukin (IL)-33.
Results
mMCP-4 reduces airway reactivity
To investigate the role of mMCP-4 in HDM-induced allergic airway inflammation, we used mMCP-4−/− mice5 and wild-type (WT) controls. Mice received intranasal doses of HDM extract twice weekly for 3 weeks. Invasive lung mechanics measurements were performed 48 h after the last challenge. HDM-treated WT and mMCP-4−/− mice both exhibited increased airway reactivity in response to inhaled methacholine as compared with phosphate-buffered saline (PBS)-treated controls (Figure 1a). However, the airway reactivity was augmented in mMCP-4−/− mice as compared with WT controls at the highest dose of metacholine used (Figure 1a), suggesting that MC chymase has a protective role by preventing excessive airway reactivity. There were no differences, regardless of treatment or genotype, in number of MCs in the lung, as determined by toluidine blue staining (Supplementary Figure S1 online).
Increased airway reactivity and airway inflammation in house-dust mite (HDM)-challenged mouse mast cell protease 4 (mMCP-4)−/− mice. Wild-type (WT) and mMCP-4−/− mice were treated with HDM extract as described in Materials and Methods. Control mice were treated with phosphate-buffered saline (PBS). (a) Forty-eight hours after the last HDM instillation, lung resistance (RL) to increasing doses of aerosolized methacholine was determined. The results from each group represent means+s.e.m.: *P<0.05; ***P<0.001 vs. corresponding values for PBS-treated mice, (*)P<0.05 vs. HDM-treated WT mice (two-way analysis of variance). Data were obtained from five independent experiments. (b–f) Number of cells in bronchoalveolar lavage fluid: total cells (b), eosinophils (c), lymphocytes (d), macrophages (e), and neutrophils (f). Data are shown as mean±s.e.m. (n=10 for controls; n=16 for HDM-treated mice). *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test). Data were obtained from seven independent experiments.
Excessive infiltration of inflammatory cells in bronchoalveolar lavage (BAL) fluid from mMCP-4−/− mice
The magnitude of the inflammatory response can be estimated by the number of inflammatory cells recruited into the tissue. Hence, we next characterized the inflammatory cells in BAL fluid. HDM-treated mice contained significantly higher number of total cells compared with untreated controls (Figure 1b). The increase in total cell numbers was predominantly reflected by a massive increase in BAL eosinophils (Figure 1c). Significant increases in BAL lymphocytes and neutrophils were also observed, whereas the BAL macrophage population was not affected (Figure 1d–f). In accordance with a protective role of chymase, higher total numbers of cells were seen in BAL fluid from HDM-treated mMCP-4−/− as compared with WT mice (Figure 1b). This was predominantly explained by effects on eosinophils, with ∼fivefold higher numbers of eosinophils found in BAL fluid from HDM-treated mMCP-4−/− mice than in WT controls (Figure 1c).
Enhanced lung tissue inflammation and ASM thickening in mMCP-4−/− mice
To further assess the role of mMCP-4 in regulating airway responses, we analyzed the lung tissue sections for signs of inflammation. As shown in Figure 2a,b, HDM treatment induced tissue inflammation in the perivascular and peribronchial areas, the inflammatory infiltrate mainly being composed of eosinophils. In line with a protective role of chymase, HDM-instilled mMCP-4−/− mice exhibited significantly higher numbers of tissue eosinophils (Figure 2c) and percentage of bronchioles surrounded with cell infiltrates (Figure 2d) than did WT controls. Next, we investigated effects on the ASM layer of the bronchi. No baseline difference in the thickness of the ASM layer was seen when comparing PBS-treated WT and mMCP-4−/− mice (Figure 2e). When WT mice were treated with HDM, there was a statistically non-significant trend towards increased ASM thickening. By striking contrast, HDM-treatment of mMCP-4−/− mice caused a profoundly increased thickening of the ASM layer compared with PBS-treated controls (Figure 2e). Hence, chymase protects against remodeling events leading to extensive thickening of the ASM layer in response to HDM. Periodic acid-Schiff staining of the lung tissue sections revealed occasional goblet cells in airways of PBS-treated mice, whereas after instillations with HDM extract, goblet cells were highly abundant (Figure 2f). However, there was no difference in the degree of goblet cell metaplasia when comparing HDM-treated WT and mMCP-4−/− mice (Figure 2f).
Histology of lungs after house-dust mite (HDM) treatment. (a and b) Hematoxylin and eosin (H&E) staining showing representative histology of primary bronchi (a) and small airways (b) from HDM-treated wild-type (WT) and mouse mast cell protease 4 (mMCP-4)−/− mice and control groups. (c) Blinded quantification of lung eosinophils (H&E staining). (d) Percentage of infiltrated small airway, as determined by blinded analysis. (e) Quantitative analysis of airway smooth muscle (ASM) thickness (H&E staining). (f) Percentage of periodic acid-Schiff (PAS)+ cells in airways. Data in c–f are shown as mean values±s.e.m. (controls; n=4–6 and HDM; n=8–14) from three independent experiments. *P<0.05; **P<0.01; ***P<0.001 (Mann–Whitney U test and Student’s t-test).
mMCP-4 regulates the IgE response
The data above indicate that mMCP-4 has an impact on the late inflammatory phase of the allergic lung disease. However, as mMCP-4-positive MCs constitute a resident cell population of the lung,6 it is conceivable that mMCP-4 can encounter HDM antigen at an early stage of the response, thereby potentially influencing the sensitization phase leading to IgE production. To address this possibility, we tested whether the absence of mMCP-4 affects the levels of serum IgE upon HDM treatment. As expected, serum IgE levels were significantly increased in HDM-treated WT mice as compared with non-treated control animals (Figure 3a). Moreover, sensitized mMCP-4−/− mice displayed significantly higher serum IgE levels as compared with WT mice (Figure 3a). These results suggest a regulatory role for mMCP-4 in the early sensitization process.
Mouse mast cell protease 4 (mMCP-4) affects the sensitization stage. (a) Concentrations of total immunoglobulin E (IgE) were measured with enzyme-linked immunosorbent assay (ELISA) in sera from house-dust mite (HDM)-treated mice and phosphate-buffered saline–treated controls. Results are expressed as mean±s.e.m. (n=3–9). Data were pooled from two independent experiments. (b–e) Increased production of interleukin (IL)-13 and IL-17A by restimulated splenocytes from HDM-treated mice. Forty-eight hours after last intranasal HDM instillation, single-cell suspensions of splenocytes were prepared. Splenocytes were restimulated with HDM extract (20 μg ml−1) for 72 h. Release of IL-13 (b), IL-17A (d) and IL-6 (e) was determined by ELISA. Results are expressed as mean±s.e.m. (n=3–7). (c) Depicts a significant positive correlation between number of recovered bronchoalveolar lavage cells and IL-13 levels in HDM-restimulated splenocyte cultures. *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test).
mMCP-4 affects cytokine release after restimulation of splenocytes
To further address the possibility that mMCP-4 affects the sensitization towards HDM antigens, we assessed whether mMCP-4 can influence the HDM-specific cytokine response. To this end, single-cell suspensions of splenocytes were restimulated with HDM extract followed by measurements of IL-13, IL-17A, and IL-6 release. As shown in Figure 3b, splenocytes from HDM-treated mMCP-4−/− mice secreted higher levels of IL-13 upon HDM restimulation than did cells from WT mice. It is also notable that the extent of IL-13 secretion by HDM-restimulated splenocytes correlated significantly with the number of BAL cells (Figure 3c). Moreover, as shown in Figure 3d, the IL-17A response was significantly higher in splenocytes from HDM-treated mMCP-4−/− mice vs. WT counterparts. By contrast, the absence of mMCP-4 did not have any effects on the secretion of IL-6 (Figure 3e). Together, these data indicate that mMCP-4 influences the activation state of the T lymphocyte populations reactive to HDM.
HDM extract induces MC degranulation and chymase release
The data above suggest that chymase, in addition to influencing the late inflammatory phase of an airway reaction, also may have effects on the sensitization phase. In turn, this implies that chymase may be released independently of crosslinking of HDM-specific IgE bound to the high affinity IgE receptors on the MC surface, e.g., through direct effects of the HDM extract on MCs. To evaluate this possibility, we determined whether HDM extract could directly induce MC degranulation leading to release of chymase. Indeed, exposure of peritoneal cell-derived MCs (PCMCs)12 to HDM extract caused significant release of both β-hexosaminidase and histamine into the supernatant, with no difference seen between WT and mMCP-4−/− PCMCs (Figure 4a,b). Moreover, significant release of chymase activity was seen when WT PCMCs were stimulated with HDM extract and as a positive control also in response to calcium ionophore (Figure 4c). Chymase activity was not detected in corresponding supernatants from mMCP-4−/− cells (Figure 4c), confirming that the chymase activity detected was attributable to mMCP-4 and not to other enzymes with overlapping substrate specificities. Taken together, these results show that MCs degranulate and release chymase in direct response to HDM.
House-dust mite (HDM) extract causes mast cell (MC) degranulation. Peritoneal cell-derived MCs (PCMCs) were stimulated with HDM extract for 1 h and (a) the percentage of β-hexosaminidase release and (b) concentration of histamine in culture supernatants were determined. (c) Chymase-like activity after HDM-stimulation of PCMCs was determined using a fluorescent substrate (Suc-Ala-Ala-Pro-Phe-AMC). Chymase-like activity is shown as raw fluorescent units (RFU) per hour. Calcium ionophore (A23187) was used as a positive control for MC degranulation. Results are shown as mean±s.e.m. (n=3). *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test). mMCP-4, mouse mast cell protease 4; WT, wild type.
Enhanced IL-33 levels in mMCP-4−/− mice
To search for mechanisms explaining the protective function of chymase in airway reactions, we evaluated the possibility that chymase acts by regulating the levels of the pro-inflammatory Th2 cytokines IL-5, IL-13, thymic stromal lymphopoietin (TSLP), and IL-33, all of these cytokines being known to be of importance in the development of allergic lung disease.9, 10 As shown in Figure 5a–c, there were no differences in the levels of IL-5, IL-13, or TSLP in the lung tissue homogenates from WT and mMCP-4−/− HDM-treated mice. By contrast, although HDM treatment did not cause any increase of lung IL-33 in WT mice above baseline levels, a profound accumulation of IL-33 was seen in lungs of HDM-treated mMCP-4−/− mice (Figure 5d). Hence, MC chymase is essential for preventing an accumulation of IL-33 in response to HDM.
Elevated interleukin (IL)-33 levels in house-dust mite (HDM)-treated mouse mast cell protease 4 (mMCP-4)−/− mice. Wild-type (WT) and mMCP-4−/− mice were treated with intranasal doses of either 3 μg HDM extract (HDM) or phosphate-buffered saline on days 1, 4, 8, 11, 15, and 18. Cytokine levels in lung homogenates were measured by enzyme-linked immunosorbent assay 4 h after last intranasal administration. Results are shown as means±s.e.m. (n=3–8). *P<0.05 (Student’s t-test). Data were pooled from three independent experiments. TSLP, thymic stromal lymphopoietin.
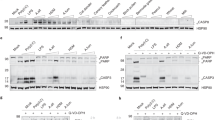
To search for the mechanism underlying the IL-33 accumulation, we evaluated the possibility that MC proteases could reduce IL-33 levels by proteolytic cleavage. To address this, we investigated the ability of WT and mMCP-4−/− PCMCs to reduce IL-33 levels. As shown in Figure 6a, IL-33 levels were rapidly reduced by MCs activated by IgE receptor crosslinking and this effect was completely blocked by a serine protease inhibitor, suggesting that the reduction of IL-33 was due to proteolytic degradation. Moreover, the effects on IL-33 were significantly diminished in cultures of mMCP-4−/− PCMCs as compared with WT cells. This suggests that MC serine proteases degrade IL-33 and that mMCP-4 participates in the process. To provide direct evidence for proteolytic effects of chymase on IL-33, recombinant murine and human IL-33 was incubated with purified mMCP-4 and human chymase, respectively, followed by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) analysis. Indeed, both mMCP-4 and human chymase caused rapid and extensive degradation of IL-33, thus confirming that IL-33 is a good substrate for MC chymase (Figure 6b). IL-33 degradation was abrogated when mMCP-4 was pre-incubated with Pefabloc SC, a general serine protease inhibitor, verifying that the observed IL-33 reduction is due to proteolysis.
Mouse mast cell protease 4 (mMCP-4) contributes to interleukin (IL)-33 degradation in vitro. (a) Wild-type (WT) and mMCP-4−/− peritoneal cell-derived mast cells were subjected to FcɛRI crosslinking (immunoglobulin E/antigen (IgE/Ag)) in the presence of exogenous IL-33. Experiments were performed with or without addition of a serine protease inhibitor (Pefabloc SC). IL-33 levels in the supernatants were measured after 20, 50, and 120 min using enzyme-linked immunosorbent assay. Data are shown as percentage of residual IL-33 compared with time=0; expressed as means±s.e.m. (n=3). *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test). (b) mMCP-4 or human chymase were incubated with murine or human IL-33, respectively. At the time points indicated, reactions were terminated followed by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and staining with Coomassie. As a control to verify that IL-33 was reduced through proteolysis, mMCP-4 was incubated for 60 min with a general serine protease inhibitor (Pefabloc SC; final concentration 2 mM) before experiments.
Discussion
Here we show that chymase, i.e., one of the major compounds secreted by activated MCs, has a major impact on HDM-induced allergic airway responses. Intriguingly, although both clinical evidence and work based on mouse models for asthma support an overall detrimental role for MCs in conjunction with asthma,13, 14, 15, 16, 17 we here demonstrate a protective role of one of the compounds secreted by activated MCs, i.e., chymase. This finding is in line with a previous study where we showed that chymase has a protective function in a model where allergic airway inflammation was induced using an OVA-based protocol,6 and also in line with previous findings showing that MC proteases, including chymase, can degrade pathogenic cytokines.18, 19, 20, 21, 22 In further support for a protective role of chymase, there is clinical evidence showing a positive correlation between chymase-positive MCs and preserved lung function in severe asthma,23, 24 and there is also clinical data suggesting that Th2-high asthma is associated with an intraepithelial MC phenotype with low chymase expression.25 Collectively, these findings thus argue that MC chymase may have a general downregulatory effect on allergic airway responses, independently of species and independently of the regimen used for provoking the response. Possibly, this could serve as a regulatory mechanism that counteracts the otherwise detrimental actions of MCs (and/or other cell types). The net impact of MCs may thus be a result of a balance between detrimental and protective actions, mediated by distinct MC-related effector mechanisms. Moreover, considering the highly heterogeneous nature of the MC populations residing in the lung,26 it cannot be excluded that beneficial and detrimental activities of MCs can be attributed to phenotypically distinct MC populations.
Our data show that HDM treatment of mice resulted in increased airway reactivity, this being in line with previous reports using similar HDM-based models.10, 11, 27 Moreover, we found evidence suggesting that the presence of chymase protects towards excessive airway reactivity at high doses of metacholine. Mechanistically, we found that the excessive airway reactivity in mMCP-4−/− mice was accompanied by a much more profound thickening of the ASM layer as compared with WT mice, ASM hypertrophy being one of the key components of airway hyperresponsiveness.28 Hence, a likely explanation for the elevated airway reactivity in mMCP-4−/− mice is that chymase prevents an expansion of the ASM layer. In addition to the effects of chymase on airway reactivity, an important finding was that the absence of chymase led to an excessive airway inflammation. In particular, chymase was shown to protect from excessive eosinophilia. Hence, the presence of chymase protects from extensive manifestations of two of the hallmark features of asthma—increased airway reactivity and eosinophilia.
To search for mechanisms operative in the protective effect of chymase in airway responses, we hypothesized that chymase may act by proteolytic effects on pro-inflammatory cytokines. This hypothesis was based on previous studies, mainly from in vitro settings, showing that chymase can cleave a variety of cytokines (reviewed in Pejler et al.3). Indeed, we show that HDM treatment resulted in a profound accumulation of IL-33 in the lung tissue of animals lacking mMCP-4, whereas no corresponding IL-33 accumulation was seen in WT mice. A likely explanation for this finding is that chymase controls IL-33 levels by proteolytic degradation of the cytokine. In support of this, WT MCs were shown to degrade IL-33 efficiently in vitro, whereas MCs lacking mMCP-4 had a reduced capacity for IL-33 degradation, and we also showed that purified chymase of both murine and human origin efficiently degrades IL-33. Interestingly, recent studies have strongly implicated IL-33 as a key cytokine regulating Th2 responses. For example, intraperitoneal administration of IL-33 in mice results in a Th2 cytokine response and increased levels of blood eosinophils, as well as enhanced IgE-levels.29, 30, 31, 32 Further, IL-33 has been linked to asthma due to its ability to provoke airway reactivity when administered in vivo33 and is emerging as a potential target in the treatment of asthma.34, 35 Together, we may thus propose that the protective effect of chymase on airway inflammation and on airway reactivity may be explained, at least partly, by effects on IL-33. Considering the central importance of IL-13 for allergic lung responses, it was somewhat unexpected that HDM treatment did not lead to increased tissue levels of IL-13. However, earlier studies have shown that an increase in IL-13 is only seen at early time points after initiation of the immunization protocol,10 whereas we used a longer immunization scheme.
In non-activated MCs, chymase is stored within the secretory granules. To have an impact on any extracellular process, it thus needs to become exocytosed. During an allergic response, this will most likely be accomplished by crosslinking of antigen-specific IgE present on the MC surface. Accordingly, chymase is expected to have effects predominantly at the late inflammatory phase of the response towards HDM immunization, i.e., at a stage when HDM-specific IgE is prevalent. However, an intriguing finding in this investigation was that the absence of chymase resulted in increased serum IgE levels in response to HDM treatment and also in an exaggerated cytokine response of HDM-restimulated splenocytes. This indicates that chymase, in addition to influencing the inflammatory phase, also can have an impact on the sensitization stage of the disease. Further, this implies that chymase may become exocytosed by mechanisms independent of HDM-specific IgE. Indeed, we show that HDM extract has direct effects on MCs, leading to exocytosis of chymase. Potentially, chymase exocytosed upon direct contact of MCs with HDM may thus have the ability to affect the initial immune response to HDM, e.g., by acting proteolytically on IL-33 or, alternatively, directly on antigens present in the HDM extract. Importantly, although our data indicate that chymase is exocytosed through a direct effect of HDM extracts on MCs, we cannot exclude that additional mechanisms of early chymase exocytosis are operative in vivo, e.g., mediated by effects of cytokines, such as IL-33.36
In summary, this study suggests that mMCP-4 is released by MCs in the local environment in response to HDM (Figure 7). mMCP-4, which is stored as an active enzyme, can thus directly act in the inflammatory process and can also be involved in regulating the initial immune response to HDM antigens. The results presented here suggest that IL-33 is one of the prime targets for exocytosed chymase and that the absence of chymase leads to an excessive accumulation of IL-33 and thereby to exaggerated airway responses.
Schematic presentation of the effects of mast cell (MC) chymase on airway responses. House-dust mite (HDM) entering the tissue may directly activate MCs leading to chymase secretion. Alternatively, chymase may be secreted in response to interleukin (IL)-33. Exocytosed chymase can regulate IL-33 levels by proteolytic degradation of the cytokine. Potentially, this may modulate IL-33-mediated effects on airway responses, including dampening of IL-33-mediated recruitment of eosinophils and IL-33-mediated stimulation of t helper type 2 (Th2) responses, leading to immunoglobulin E (IgE) production. APC, antigen-presenting cells; mMCP-4, mouse mast cell protease 4.
Methods
Mice and model of allergic airway inflammation. mMCP-4−/− mice5 backcrossed to the C57BL/6J strain for 13 generations and WT littermate controls were used. Eight-week-old male mice were lightly anesthetized with isoflurane and instilled intranasally with 3 μg HDM extract from species Dermatophagoides farinae (Greer Laboratories, Lenoir, NC), dissolved in 30 μl PBS, on days 1, 4, 8, 11, 15, and 18. Control mice were instilled with PBS intranasally. All animal experiments were approved by the local ethical committee.
Measurement of airway reactivity. Forty-eight hours after the final instillation, mice were anesthetized with pentobarbital sodium (50 mg kg−1; Sigma-Aldrich, St Louis, MO), tracheostomized, and intubated to a small animal ventilator (FinePointe, Buxco, Winchester, UK). Mice were mechanically ventilated at 160 breaths min−1 with a tidal volume of 0.25 ml. Before measurements, mice were shortly ventilated to reach an acclimatization baseline. Lung function was assessed with a dose–response curve to increasing doses of aerosolized methacholine (2–16 mg ml−1; Sigma-Aldrich) via the tracheal cannula. Lung resistance (RL) is shown as percentage of change from baseline. Results were pooled from five separate and matched experiments.
BAL and lung tissue sampling and homogenization. BAL fluid was collected as previously described.37 Cytospin slides were prepared and the percentage of leukocyte populations was determined after May Grünwald/Giemsa staining. For cytokine measurements, the right lung lobes were frozen on dry ice and stored at −70 °C. Lung tissue was homogenized in HBSS (Hank’s balanced salt solution) containing protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) at a concentration of 50 mg lung tissue per ml buffer. The homogenates were centrifuged at 800 × g at 4 °C for 8 min, and supernatants were collected for cytokine measurements using enzyme-linked immunosorbent assay (ELISA).
Histology. The left lung lobe was preserved in 4% paraformaldehyde, paraffin-embedded, sectioned and stained with hematoxylin and eosin (H&E) as previously described.6, 38 The extent of tissue inflammation around the primary bronchi was evaluated by blinded counting of eosinophils. In addition, broncho-vascular units containing cell infiltration were counted, and results are shown as percentage per individual. Thickness of the ASM layer around similar size bronchi (diameter range 500–650 μm) was measured with a Nikon Microphot-FXA microscope using the × 10 objective lens (Bergström Instrument AB, Stockholm, Sweden) and Eclipse Net software (version 1.20, developed by Laboratory Imaging, Prague, Czech Republic) on H&E-stained sections. ASM layer thickness was measured every 30 μm around the primary bronchi, and average thickness was calculated. For periodic acid-Schiff staining, slides were deparaffinized, hydrated, oxidized in 0.05% periodic acid for 5 min, washed in H2O, stained with Schiff’s reagent (Sigma-Aldrich) for 30 min, washed in H2O for 3 min, and counterstained with Mayer’s hematoxylin for 30 s. MCs were identified by toluidine blue staining.39
Restimulation of splenocytes. Restimulation of splenocytes was performed as previously described.40 Forty-eight hours after the last intranasal instillation, the spleens were collected and mechanically dispersed followed by passing of the tissue through a nylon filter. Red blood cells were eliminated by lysis. Splenocytes were seeded (4 × 106 cells ml−1, 1 ml per well) into a 24-well plate and were stimulated with 20 μg ml−1 HDM extract for 72 h. ELISA was used to measure release of cytokines into the cell culture supernatant.
Generation and degranulation of PCMCs. PCMCs were established according to a previously described protocol.12 IgE anti-TNP (anti-trinitrophenyl; BD Biosciences Pharmingen, Stockholm, Sweden) was added to 1 × 106 PCMCs at 1 μg ml−1 followed by incubation overnight at 37 °C. Cells were washed three times and resuspended in Dulbecco’s modified Eagle’s medium supplemented as described above. OVA-TNP was added (0.4 μg ml−1 final concentration). For calcium ionophore-mediated degranulation, A23187 (Sigma-Aldrich) was added (2 μM final concentration). Mouse recombinant IL-33 (PeproTech, London, UK) was added at 2 ng ml−1 immediately after stimulation with IgE anti-TNP. To block serine protease activity, 1 mM Pefabloc SC (Pentapharm LTD, Basel, Switzerland) was added 10 min before MC activation. Supernatants were collected 0, 20, 40, and 60 min after stimulation.
Chymase activity. PCMCs were washed twice and resuspended in HBSS at a density of 1 × 106 cells ml−1 and placed in triplicates (100 μl per well) in a 96-well plate. HDM extract was added at a final concentration of 200 μg ml−1. A23187 (2 μM final concentration) was used as a positive control for PCMC degranulation. Chymase-like activity was measured using a fluorogenic substrate (Suc-Ala-Ala-Pro-Phe-AMC), according to the manufacturer’s instructions (Bachem, Weil am Rhein, Germany). The reaction was allowed to proceed for 2 h in room temperature, and fluorescence was measured at 360 nm (excitation) and 460 nm (emission) every 10 min using a FARCyte microplate reader (Amersham Biosciences, Uppsala, Sweden).
IL-33 degradation. Human chymase (Sigma-Aldrich, Stockholm, Sweden; 100 ng) or purified mMCP-4 (1 ng)41 were incubated at 37 °C with recombinant human (PeproTech) or murine (PeproTech) IL-33 (1 μg per lane) in PBS (total volume 20 μl). At different time points, 5 μl of 5 × SDS-PAGE sample buffer was added, followed by SDS-PAGE (12% gels), and visualization of protein bands by Coomassie staining.
ELISA and β-hexosaminidase release assay. Concentrations of total IgE in serum were measured using ELISA (Bethyl Laboratories, Montgomery, TX). ELISA was used to determine the levels of IL-5, IL-6, IL-13, IL-17A, IL-33, and TSLP, according to the manufacturer’s instructions (eBioscience, San Diego, CA). Histamine release was measured with ELISA (EA31; Oxford Biomedical Research, Oxford, MI). Release of β-hexosaminidase was determined as previously described.39
Statistical analysis. Statistical significances were calculated by Student’s t-test for differential counting and ELISA; Mann–Whitney U test for histology; two-way analysis of variance for airway reactivity measurements. GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) was used for all statistical analyses. All values are displayed as means±s.e.m. and P-values <0.05 were considered to be significant.
References
Galli, S.J. & Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 18, 693–704 (2012).
Lundequist, A. & Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 68, 965–975 (2011).
Pejler, G., Ronnberg, E., Waern, I. & Wernersson, S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115, 4981–4990 (2010).
Boyce, J.A. Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem. Immunol. Allergy 87, 59–79 (2005).
Tchougounova, E., Pejler, G. & Abrink, M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J. Exp. Med. 198, 423–431 (2003).
Waern, I. et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 183, 6369–6376 (2009).
Bates, J.H., Rincon, M. & Irvin, C.G. Animal models of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L401–L410 (2009).
Boyce, J.A. & Austen, K.F. No audible wheezing: nuggets and conundrums from mouse asthma models. J. Exp. Med. 201, 1869–1873 (2005).
Galli, S.J., Tsai, M. & Piliponsky, A.M. The development of allergic inflammation. Nature 454, 445–454 (2008).
Gregory, L.G. et al. Inhaled house dust mite induces pulmonary T helper 2 cytokine production. Clin. Exp. Allergy 39, 1597–1610 (2009).
Johnson, J.R. et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169, 378–385 (2004).
Malbec, O. et al. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J. Immunol. 178, 6465–6475 (2007).
Yu, M. et al. Identification of an ifn-gamma/mast cell axis in a mouse model of chronic asthma. J. Clin. Invest. 121, 3133–3143 (2011).
Yu, M., Tsai, M., Tam, S.Y., Jones, C., Zehnder, J. & Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Invest. 116, 1633–1641 (2006).
Brightling, C.E., Bradding, P., Symon, F.A., Holgate, S.T., Wardlaw, A.J. & Pavord, I.D. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 346, 1699–1705 (2002).
Amin, K., Janson, C., Boman, G. & Venge, P. The extracellular deposition of mast cell products is increased in hypertrophic airways smooth muscles in allergic asthma but not in nonallergic asthma. Allergy 60, 1241–1247 (2005).
Andersson, C.K., Bergqvist, A., Mori, M., Mauad, T., Bjermer, L. & Erjefält, J.S. Mast cell-associated alveolar inflammation in patients with atopic uncontrolled asthma. J. Allergy Clin. Immunol. 127, 905–912 e901–907 (2011).
Waern, I. et al. Mast cells limit extracellular levels of IL13 via a serglycin proteoglycan-serine protease axis. Biol. Chem. 393, 1555–1567 (2012).
Akahoshi, M. et al. Mast cell chymase reduces the toxicity of gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Invest. 121, 4180–4191 (2011).
Piliponsky, A.M. et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am. J. Pathol. 181, 875–886 (2012).
Zhao, W., Oskeritzian, C.A., Pozez, A.L. & Schwartz, L.B. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J. Immunol. 175, 2635–2642 (2005).
Sugimoto, K. et al. The alphavbeta6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J. Clin. Invest. 122, 748–758 (2012).
Balzar, S., Chu, H.W., Strand, M. & Wenzel, S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am. J. Respir. Crit. Care Med. 171, 431–439 (2005).
Zanini, A. et al. Chymase-positive mast cells play a role in the vascular component of airway remodeling in asthma. J. Allergy Clin. Immunol. 120, 329–333 (2007).
Dougherty, R.H. et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(h)2-high asthma. J. Allergy Clin. Immunol. 125, 1046–1053 e1048 (2010).
Andersson, C.K., Mori, M., Bjermer, L., Lofdahl, C.G. & Erjefalt, J.S. Novel site-specific mast cell subpopulations in the human lung. Thorax 64, 297–305 (2009).
Yu, C.K. & Chen, C.L. Activation of mast cells is essential for development of house dust mite dermatophagoides farinae-induced allergic airway inflammation in mice. J. Immunol. 171, 3808–3815 (2003).
An, S.S. et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur. Respir. J. 29, 834–860 (2007).
Smithgall, M.D., Comeau, M.R., Yoon, B.R., Kaufman, D., Armitage, R. & Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, INKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Stolarski, B., Kurowska-Stolarska, M., Kewin, P., Xu, D. & Liew, F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 185, 3472–3480 (2010).
Kurowska-Stolarska, M. et al. IL33 induces antigen-specific IL5+ T cells and promotes allergic-induced airway inflammation independent of IL4. J. Immunol. 181, 4780–4790 (2008).
Schmitz, J. et al. Il-33, an interleukin-1-like cytokine that signals via the IL1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Kondo, Y. et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20, 791–800 (2008).
Liu, X., Li, M., Wu, Y., Zhou, Y., Zeng, L. & Huang, T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 386, 181–185 (2009).
Sakashita, M. et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with japanese cedar pollinosis. Clin. Exp. Allergy 38, 1875–1881 (2008).
Pushparaj, P.N. et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. USA 106, 9773–9778 (2009).
Waern, I. et al. Accumulation of ym1 and formation of intracellular crystalline bodies in alveolar macrophages lacking heparanase. Mol. Immunol. 47, 1467–1475 (2010).
Braga, T., Grujic, M., Lukinius, A., Hellman, L., Abrink, M. & Pejler, G. Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells. Biochem. J. 403, 49–57 (2007).
Rönnberg, E. & Pejler, G. Serglycin: the master of the mast cell. Methods Mol. Biol. 836, 201–217 (2012).
Lundequist, A. et al. Prostaglandin e(2) exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J. Immunol. 184, 433–441 (2010).
Pejler, G. & Karlstrom, A. Thrombin is inactivated by mast cell secretory granule chymase. J. Biol. Chem. 268, 11817–11822 (1993).
Acknowledgements
This work was supported by grants from the Swedish Research Council, the Vårdal Foundation, the Swedish Society of Medicine, the Konsul Th C Bergh Foundation, the Åke Wiberg Foundation, the Bror Hjerpstedt Foundation, and from the Lennander Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Waern, I., Lundequist, A., Pejler, G. et al. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol 6, 911–920 (2013). https://doi.org/10.1038/mi.2012.129
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.129
This article is cited by
-
Bronchial eosinophils, neutrophils, and CD8 + T cells influence asthma control and lung function in schoolchildren and adolescents with severe treatment-resistant asthma
Respiratory Research (2022)
-
Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage
Scientific Reports (2018)
-
Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons
Cell Death & Disease (2018)
-
Potential effector and immunoregulatory functions of mast cells in mucosal immunity
Mucosal Immunology (2015)
-
Mast cell secretory granules: armed for battle
Nature Reviews Immunology (2014)