Abstract
This study identified a novel phenomenon that dendritic cells (DCs) produced interleukin (IL)-33 via Toll-like receptor (TLR)-mediated innate pathway. Mouse bone marrow–derived DCs were treated with or without microbial pathogens or recombinant murine IL-33. IL-33 mRNA and protein were found to be expressed by DCs and largely induced by several microbial pathogens, highly by lipopolysaccharide (LPS) and flagellin. Using two mouse models of topical challenge by LPS and flagellin and experimental allergic conjunctivitis, IL-33-producing DCs were observed in ocular mucosal surface and the draining cervical lymph nodes in vivo. The increased expression levels of myeloid differentiation primary-response protein 88 (MyD88), nuclear factor (NF)-κB1, NF-κB2, and RelA accompanied by NF-κB p65 nuclear translocation were observed in DCs exposed to flagellin. IL-33 induction by flagellin was significantly blocked by TLR5 antibody or NF-κB inhibitor quinazoline and diminished in DCs from MyD88 knockout mice. IL-33 stimulated the expression of DC maturation markers, CD40 and CD80, and proallergic cytokines and chemokines, OX40L, IL-4, IL-5, IL-13, CCL17 (C-C motif chemokine ligand 17), TNF-α (tumor necrosis factor-α), and IL-1β. This stimulatory effect of IL-33 in DCs was significantly blocked by ST2 antibody or soluble ST2. Our findings demonstrate that DCs produce IL-33 via TLR/NF-κB signaling pathways, suggesting a molecular mechanism by which local allergic inflammatory response may be amplified by DC-produced IL-33 through potential autocrine regulation.
Similar content being viewed by others
Introduction
Interleukin-33 (IL-33), a new member of the IL-1 super family, has been recently identified as a functional ligand to ST2. By binding to ST2 receptor, IL-33 activates T helper type 2 (Th2) cells and mast cells to secrete Th2 cell–associated proinflammatory cytokines and chemokines that lead to allergic pathological changes in mucosal organs.1 More and more evidences show that IL-33 has an important role in inflammatory diseases: hypersensitive diseases like asthma,2, 3 autoimmune diseases like rheumatoid arthritis,4 dermatitis,5, 6 colitis,7, 8, 9 allergic rhinitis,10 and conjunctivitis.11, 12 Major sources of IL-33 expression include epithelial and endothelial cells, as well as fibroblasts and others.1, 2, 13, 14, 15 Until recently, Yanagawa and colleagues reported that murine bone marrow–derived dendritic cells (DCs) could express IL-33.16, 17
DCs are the most potent professional antigen-presenting cells linking innate and adaptive immune response. DCs express a variety of Toll-like receptors (TLRs), which recognize conserved microbial components and have an important role in the mucosal innate immune system.18 Mucosal surfaces contain resident DCs capable of sensing the external stimuli and mounting local responses upon recognition of invading microorganisms.19 We have demonstrated that IL-33 expression is an innate response to certain microbial pathogens through TLRs and nuclear factor (NF)-κB signaling pathways in human corneal epithelial cells.20 However, there is no report that DCs produce IL-33 via TLR-mediated innate immunity signaling. As in most tissues, ocular surface DCs are present at very low concentrations and are difficult to isolate.21 DCs induced from mouse bone marrow have been widely used for the study.22, 23 Using both in vitro cultured bone marrow–derived DCs and in vivo ocular surface of BALB/c mice topically challenged with microbial pathogens, the present study explored a novel phenomenon that DCs produce IL-33 via TLR-mediated innate signaling pathway in response to microbial pathogens, which may have an important role in amplifying the allergic inflammatory response in local mucosa through a potential autocrine regulatory mechanism.
Results
IL-33 was produced by mouse DCs in response to specific TLR ligands
Mouse DCs were induced from bone marrow cells by GM-CSF (granulocyte macrophages colony-stimulating factor) in culture for 9 days, and the purity of DCs was >92%, as evaluated by DC surface marker CD11c with flow cytometry analysis. The DCs were treated with or without extracted or synthetic microbial products that are ligands to TLRs 1–9 (10 μg ml−1 of Pam3CSK4, peptidoglycan (PGN), flagellin, diacylated lipoprotein (FSL-1), R837, single-stranded RNA (ssRNA), type C CpG oligonucleotide (C-CpG-ODN), 1 μg ml−1 of lipopolysaccharide (LPS), or 50 μg ml−1 of Polyinosinicpolycytidylic acid (polyI:C)) for 4–24 h. IL-33 mRNA was found to be expressed at very low level in untreated DCs, but it was largely induced by specific TLR ligands (Figure 1a). The induction of IL-33 mRNA expression in DCs reached the peak level at 8 h (Figure 1b). As shown in Figure 1a,b, LPS and flagellin significantly induced IL-33 mRNA expression to 6–10-fold (P<0.01) in a dose-dependent fashion; Pam3CSK4 and poly I:C also upregulated IL-33 mRNA by 3–4-fold (P<0.05); whereas PGN, FSL-1, R837, ssRNA, and C-CpG-ODN did not significantly induce IL-33 expression.
Dendritic cells (DCs) produce interleukin (IL)-33 in response to microbial pathogens. (a) IL-33 mRNA expression was determined by quantitative real-time PCR in murine DCs from BALB/c mice exposed to microbial products, ligands to Toll-like receptors 1–9 (10 μg ml−1 of Pam3CSK4, peptidoglycan (PGN), flagellin, diacylated lipoprotein (FSL-1), R837, single-stranded RNA, type C CpG oligonucleotide (C-CpG-ODN), 1 μg ml−1 of lipopolysaccharide (LPS) or 50 μg ml−1 of polyinosinicpolycytidylic acid (polyI:C)) for 8 h. (b) The time course and dose response of IL-33 mRNA by DCs exposed to LPS or flagellin. (c, d) IL-33 protein production in cell lysates of DCs treated with LPS (1 μg ml−1) or flagellin (10 μg ml−1) for 24 h was determined by enzyme-linked immunosorbent assay and western blotting, respectively. (e) Representative images showing IL-33 immunoreactivity (green) in DCs exposed to LPS (1 μg ml−1) or flagellin (10 μg ml−1) for 24 h by immunofluorescent staining (Green) with propidium iodide (Red) as nuclear counterstaining. Each bar in the diagrams represents mean±s.d. of three to five independent experiments. *P<0.05; **P<0.01. MW, molecular weight. The color reproduction of this figure is available on Mucosal Immunology journal online.
To confirm the IL-33 production at protein level, DCs were exposed to LPS and flagellin for 24 h, which were microbial ligands to TLRs 4 and 5, respectively, and strongly stimulated the IL-33 protein expression. IL-33 protein was barely detected in the cell lysates from untreated DCs but was significantly stimulated to 2–3-fold by LPS and flagellin (P<0.05), as determined by enzyme-linked immunosorbent assay (ELISA; Figure 1c) and western blotting (Figure 1d). The immunofluorescent staining further showed that IL-33 was immunolocalized in the nucleus and cytoplasma in normal DCs, and IL-33-positive cells were largely increased with more significant cytoplasmic immunostaining in DCs treated with LPS or flagellin (Figure 1e).
Furthermore, we observed that LPS and flagellin significantly upregulated DC maturation markers, CD40, CD80, CD86, and MHC (major histocompatibility complex) class II, with more and large clumps formed in DC cultures, indicating that DC maturation may contribute to IL-33 induction by microbial ligands (see Supplementary Figure S1 online).
IL-33 was produced by CD11c+ DCs infiltrated in conjunctiva and migrated to cervical lymph nodes (CLNs) of mice topically challenged by LPS or flagellin
To further identify whether DCs produce IL-33 in vivo, LPS (1 μg per 5 μl phosphate-buffered saline (PBS) per eye) or flagellin (1 μg per 5 μl PBS per eye) was topically instilled in the conjunctival sac of BALB/c mice three times a day for 2 days. The mice treated with 5 μl PBS alone were used as controls. As shown in Figure 2, the double immunofluorecent staining revealed that murine corneal and conjunctival epithelia weakly expressed IL-33 in normal control mice but produced strong IL-33 immunoreactivity when stimulated by LPS or flagellin, indicating that IL-33 is mainly produced by epithelial cells, a similar pattern to human ocular surface epithelia.20 Interestingly, IL-33 immunoreactivity was also observed in CD11c+ DCs that infiltrated into the conjunctival stroma near epithelial area (Figure 2a) and draining CLNs (Figure 2b) in the mice challenged with LPS or flagellin. This finding further identified that DCs produce IL-33 in vivo in murine mucosal ocular surface and draining CLNs in response to microbial pathogens.
Mucosal dendritic cells (DCs) produce interleukin (IL)-33 in murine conjunctiva (Conj) and cervical lymph nodes (CLN) in vivo. BALB/c mice were topically challenged in the conjunctival sac with 1 μg lipopolysaccharide (LPS) or flagellin in 5 μl phosphate-buffered saline (PBS) per eye, three times a day for 2 days, 5 μl PBS was used as a control. Frozen sections of eyeballs ((a) showing cornea and conjunctiva) and (b) CLN were used for double immunofluorescent staining with CD11c (Red) and IL-33 (Green) using DAPI (4′,6-diamidino-2-phenylindole) counterstaining (Blue). Arrows indicate positive staining signals. Each bar in the diagrams represents mean±s.d. of three independent experiments. *P<0.05; **P<0.01.
IL-33-producing DCs in murine experimental allergic conjunctivitis (EAC)
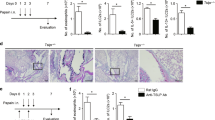
To confirm the role of IL-33-producing DCs in allergic disease, EAC model was induced in BALB/c mice sensitized and topically challenged by short ragweed (SRW) pollen (EAC mice), with PBS treated (PBS mice) as control groups. Repeated topical challenges with SRW allergen generated typical signs mimic to human allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, and frequent scratching of the eye lids. Consistent with our previous reports,24, 25 the infiltration of CD11c+ DCs on the ocular surface was detected in this EAC model by immunostaining. As shown in Figure 3, CD11c+ DCs were accumulated in the SRW-challenged ocular surface, primarily in the stroma subjacent to conjunctival epithelia. Interestingly, double staining showed some DCs producing IL-33 (Figure 3a). In draining CLN, we also observed the dramatic increase of positively immunoreactive cells to both CD11c and IL-33 (Figure 3b). These results suggest a potential role of IL-33-expressing DCs in EAC mice.
Interleukin (IL)-33-producing dendritic cells in murine experimental allergic conjunctivitis (EAC). The EAC model was induced in BALB/c mice sensitized and topically challenged by SRW pollen (EAC), with phosphate-buffered saline (PBS)-treated mice (PBS) as controls. Frozen sections of eyeballs ((a) showing cornea and conjunctiva) and (b) cervical lymph nodes (CLNs) were used for double immunofluorescent staining with CD11c (Red) and IL-33 (Green) using DAPI (4′,6-diamidino-2-phenylindole) counterstaining (Blue). Arrows indicate positive staining signals. Each bar in the diagrams represents mean±s.d. of three independent experiments. *P<0.05; **P<0.01.
IL-33 induction was mediated via TLR/NF-κB signaling pathways by flagellin
Myeloid differentiation primary-response protein 88 (MyD88) is a universal adapter protein necessary for response to most TLRs except TLR3.26, 27 TLR signaling typically induces activation of the NF-κB. Therefore, we hypothesized that LPS and flagellin promoted IL-33 production via TLR/NF-κB signaling pathways. Taking flagellin as an example, we observed the significant increase of MyD88, NF-κB1, and NF-κB2 (all P<0.01), as well as RelA (P<0.05) that encodes NF-κB p65, at mRNA levels, in the DCs exposed to flagellin (Figure 4a). Immunofluorescent staining revealed that NF-κB p65 protein was mainly located in cytoplasm in untreated control DCs but was markedly translocalized from cytoplasm to nucleus in DCs exposed to flagellin (Figure 4b), indicating NF-κB signaling activation. Interestingly, the stimulated mRNA expression of MyD88, NF-κB1, and NF-κB2, the nuclear translocation of NF-κB p65, and the increased IL-33 production by flagellin were significantly blocked by TLR5 antibody or quinazoline, a NF-κB activation inhibitor (NF-κB-I), with an exception that NF-κB-I did not block the stimulated MyD88 mRNA, an upstream molecule of NF-κB (Figure 4).
Interleukin (IL)-33 is induced in dendritic cells (DCs) via Toll-like receptor (TLR)/nuclear factor (NF)-κB signaling pathways. (a) The mRNA expression levels of MyD88 (myeloid differentiation primary-response protein 88), NF-κB1, NF-κB2, RelA, and IL-33 were evaluated by quantitative real-time PCR in DCs from BALB/c mice treated with flagellin (10 μg ml−1) with or without TLR5 antibody or quinazoline (NF-κB-I) 1 h preincubation for 8 h. (b) Representative images showing NF-κB p65 nuclear translocation and IL-33 production by immunofluorescent staining (Green) with propidium iodide counterstaining (Red) in DCs treated as described in a for 24 h. *P<0.05; **P<0.01, compared with control; ^P<0.05; ^^P<0.01, compared with flagellin. The color reproduction of this figure is available on Mucosal Immunology journal online.
Furthermore, we cultured bone marrow–derived DCs from MyD88−/− mice and their age- and gender-matched wild-type MyD88+/+ littermates to investigate whether MyD88 signaling is essential for TLR activation and IL-33 induction. As shown in Figure 5a, the mRNA expression levels of NF-κB signaling (NF-κB1, NF-κB2, RelA) and IL-33 induction (P<0.01, 0.01, 0.05 and 0.01, respectively) were strongly stimulated by flagellin in DCs derived from MyD88+/+ mice when compared with the untreated control. But these stimulatory effects of flagellin were largely abolished in DCs derived from MyD88−/− mice. IL-33 induction, evaluated by its immunoreactivity (Figure 5b), was also significantly increased by flagellin in DCs of MyD88+/+ wild type but not MyD88−/− mice.
Interleukin (IL)-33 is induced in dendritic cells (DCs) via MyD88 (myeloid differentiation primary-response protein 88) signaling pathways. (a) The mRNA expression of nuclear factor (NF)-κB1, NF-κB2, RelA, and IL-33 was evaluated by quantitative real-time PCR in DCs from MyD88+/+ and MyD88−/− mice exposed to flagellin (10 μg ml−1) for 8 h. (b) Representative images showing IL-33 production by immunofluorescent staining (Green) with propidium iodide counterstaining (Red) in DCs from MyD88+/+ and MyD88−/− mice treated with flagellin (10 μg ml−1) for 24 h. Each bar in the diagrams represents mean±s.d. of three independent experiments. *P<0.05; **P<0.01, compared with MyD88+/+ control, ^P<0.05; ^^P<0.01, compared with MyD88+/+ flagellin.
Potential autocrine regulation of IL-33/ST2 in DCs activation
DCs have an important role in initiating and maintaining the Th2-dominant allergic inflammation.28 IL-33 has been identified as the ligand of ST2, the well-known receptor in Th2 cells.1 Through binding to ST2, IL-33 promotes Th2 cells producing Th2 inflammatory cytokines IL-4, IL-5, and IL-13 in allergic disease. Based on a recent report that ST2 was identified to be expressed by DCs,23 our finding that IL-33 is produced by DCs in response to microbial pathogens leads us to hypothesize that DCs may amplify allergic inflammatory response via potential autocrine regulation in IL-33/ST2 signaling.
Mouse DCs were incubated with 1 ng ml−1 of recombinant murine (rm) IL-33 for 4 or 24 h. As evaluated by quantitative real-time PCR (RT-qPCR) and flow cytometry, we found that IL-33 increased the expression of CD40 and CD80 at both mRNA and protein levels (P<0.05; Figure 6a,b), suggesting that IL-33 may have a role in promoting DC maturation. We have observed that IL-33 activated DCs to produce Th2 cytokines (IL-4, IL-5, and IL-13), Th2-attracting chemokine CCL17 (C-C motif chemokine ligand 17), and proinflammatory cytokines (TNF-α (tumor necrosis factor-α) and IL-1β; Figure 6a). More interestingly, we observed a previously unknown phenomenon that IL-33 primes DCs to produce Th2-inducing cytokine OX40 ligand (OX40L) at both mRNA and protein levels (Figure 6a,b), which is consistent with a recent study.29 OX40L is well known to be induced in activated DCs by thymic stromal lymphopoietin, an epithelial-derived proallergic cytokine, to trigger Th2-dominant allergic inflammatory response. Interestingly, the stimulated expression of DC maturation markers (CD40 and CD80) and proallergic cytokines and chemokines (OX40L, IL-4, IL-5, IL-13, CCL17, TNF-α, and IL-1β) by IL-33 was significantly blocked by ST2 neutralizing antibody or exogenous soluble ST2 protein. These results demonstrate that DCs may have an important role in amplifying local allergic inflammation through a potential autocrine regulation of IL-33/ST2 signaling.
Interleukin (IL)-33 activates dendritic cells (DCs) in vitro. (a) The expression of cytokines and chemokines was evaluated by quantitative real-time PCR in DCs from BALB/c mice exposed to IL-33 (1 ng ml−1) for 4 h without or with 1 h previous incubation of ST2 antibody (Ab) or soluble ST2 protein. (b) Flow cytometry showed the enhanced expression of CD40, CD80, and OX40L of DCs exposed to IL-33 for 24 h. Each bar in the diagrams represents mean±s.d. of three independent experiments. *P<0.05; **P<0.01, ***P<0.001, compared with control; ^P<0.05, compared with IL-33. APC, allophycocyanin; CCL, C-C motif chemokine ligand; FITC, fluorescein isothiocyanate; MFI, mean fluorescent intensity; PE, phycoerythrin; SSC-A, side scatter area; TNF, tumor necrosis factor.
Discussion
IL-33 has been consolidated as a pro-inflammatory mediator in allergic inflammation.1, 30, 31, 32 IL-33 signals through a heterodimeric membrane receptor composed of ST2 and IL-1R1 accessory protein and activates Th2 lymphocytes, mast cells, eosinophils, basophils, macrophages, NK (natural killer), and NKT cells.33, 34 The environmental or endogenous triggers that provoke IL-33 cellular release may be associated with infection, inflammation, or tissue damage.2 Relatively abundant IL-33 mRNA expression is found in multiple tissue–related cell types such as mucosal epithelial cells, endothelial cells, fibroblasts, smooth muscle, cardiomyocytes, keratinocytes, and adipocytes.1, 13, 35 Skin, gut, and lung appear to be prominent IL-33-expressing organs. However, whether DCs express IL-33 is not clear, although one group has reported recently that DCs produce IL-33 during inflammation.16, 17 The involvement of bacterial agents like LPS and flagellin in initiation of allergic inflammation has been recently documented.36, 37, 38, 39, 40, 41 In this study, we demonstrated that IL-33 was induced by mouse DCs, possibly via TLR/NF-κB signaling pathways in response to microbial pathogens. The DC-produced IL-33 may amplify local inflammatory response through autocrine mechanism.
DCs produce IL-33 both in vitro and in vivo in response to microbial pathogens
Based on the important role of DCs in innate immunity and the observation that IL-33 is mainly produced by epithelial cells via TLR-mediated innate response,20 we hypothesized that DCs are capable of producing IL-33 in response to microbial pathogens. We incubated the murine bone marrow–derived DCs with TLR ligands 1–9 and found that several TLR ligands, especially LPS and flagellin, the ligands to TLR4 and TLR5, respectively, significantly stimulated IL-33 expression by mouse DCs at both the mRNA (P<0.01) and protein levels (P<0.05), as determined by RT-qPCR, ELISA, western blotting, and immunofluorescent staining (Figure 1). DCs are highly mobile and are present in the right place at the right time for the regulation of immunity. They are positioned as sentinels in the periphery, where they frequently encounter foreign antigens and penetrate epithelium to sample antigens,19 then they readily relocate to secondary lymphoid organs, particularly lymph nodes, to position themselves optimally for encounter with naive or central memory T cells.42, 43 Using a topical challenge mouse model with LPS and flagellin, we further identified that DCs produce IL-33 in vivo. The infiltrated CD11c+ DCs that produce IL-33 were observed in the conjunctiva of LPS- or flagellin-challenged eyes, as evaluated by immunofluorescent staining (Figure 2). Interestingly, we further found that CD11c+ DCs migrated to CLNs and expressed high level of IL-33. The double-reactive cells (IL-33+CD11c+), the most should be DCs, in CLN significantly increased 3.7–4.5-fold, from 4.14±1.26‰ in control mice to 18.64±3.05 and 15.28±2.52‰ in LPS- or flagellin-challenged mice, respectively. Furthermore, IL-33-producing DCs were observed to accumulate in the ocular surface and the draining lymph nodes in a murine EAC model, as evaluated by double staining with CD11c and IL-33 antibodies (Figure 3). The double-reactive cells (IL-33+CD11c+) in CLN significantly increased 5.2-fold, from 3.87±0.61‰ in PBS control mice to 20.24±3.12‰ in EAC mice.
DCs produce IL-33 via TLR/NF-κB signaling pathways
LPS- and flagellin-induced inflammation is mediated by TLR4 and TLR5, respectively.44, 45 TLRs, which recognize conserved microbial components, are important pattern-recognition receptors. TLRs consist of a family of at least 11 mammalian receptors that bind a restricted repertoire of ligands and recruit common adapter molecules to induce cell signaling.46 MyD88 is a universal adapter protein necessary for response to most TLRs, including TLR4 and TLR5.26, 27 TLR signaling typically induces activation of NF-κB. NF-κB1 or NF-κB2 is bound to RelA to form the NF-κB complex. Transcription factor p65 is encoded by the RelA gene.47 Activated NF-κB complex translocates into the nucleus and binds DNA at kappa-B-binding motifs. Using flagellin as a model, we observed that flagellin significantly increased the expression of MyD88, NF-κB1, NF-κB2, RelA, and IL-33, as well as NF-κB activation with p65 nuclear translocation (Figure 4). The stimulated IL-33 induction by flagellin was markedly blocked by TLR5 antibody and NF-κB inhibitor. Furthermore, we observed that the IL-33 induction by flagellin was significantly reduced in DCs derived from MyD88 knockout mice when compared with that seen in their wild-type littermates. All these results demonstrated that IL-33 production in DCs was via TLR/NF-κB signaling pathways in response to microbial pathogens.
DCs may amplify local inflammatory response through a potential autocrine mechanism
DCs have an important role in initiating and maintaining an allergic Th2 immune response.28 Mucosal epithelium–derived IL-33 can activate Th2 cells and mast cells to produce Th2 inflammatory cytokines and chemokines through binding to ST2 receptor.1 As DCs could express ST2,23 our finding that DCs also produce IL-33 suggest that DCs may amplify inflammatory response via potential autocrine mechanism. In this study, we observed that exogenous IL-33 stimulated expression of costimulatory molecules CD40, CD80, and OX40L, as well as Th2 inflammatory cytokines and chemokines in DCs. Furthermore, these stimulatory effects of IL-33 were significantly blocked by ST2 antibody or soluble ST2 protein. The role of IL-33-producing DCs was further observed in an EAC mouse model (Figure 3). DC-produced IL-33 may serve as a source of IL-33 for other immune cells expressing ST2, potentially amplifying allergic inflammation through a paracrine mechanism.33, 34
In summary, this study revealed that microbial pathogens, like LPS and flagellin, induce expression and production of pro-allergic cytokine IL-33 by DCs via TLR/NF-κB signaling pathways. DCs not only respond to IL-33 but also produce IL-33 in allergic condition. Our findings suggest a novel mechanism by which local inflammatory response may be amplified by DC-produced IL-33 through a potential autocrine mechanism, which may provide a therapeutic potential to treat ocular allergic disease through a local blockade of IL-33 produced by DCs.
Methods
Materials and reagents. Cell culture dishes, plates, centrifuge tubes, and other plasticware were purchased from BD Biosciences (Lincoln Park, NJ); polyvinylidene difluoride (PVDF) membrane was from Millipore (Bedford, MA); polyacrylamide ready gels (4–15% Tris-HCl), sodium dodecyl sulfate (SDS), prestained SDS-polyacrylamide gel electrophoresis low range standards, precision plus protein standards, and precision protein Strep-Tactin horseradish peroxidase conjugate were from Bio-Rad (Hercules, CA); RPMI-1640 medium, amphotericin B and gentamicin were from Invitrogen (Grand Island, NY); fetal bovine serum from Hyclone (Logan, UT). The extracted or synthetic microbial components, Pam3CSK4, PGN from Bacillus subtilis (PGN-BS), flagellin from Salmonella typhimurium, FSL-1, imiquimod (R837), single-stranded, GU-rich oligonucleotide complexed with LyoVec (ssRNA40/LyoVec), and type C CpG oligonucleotide (ODN 2395) were from invivoGen (Santiago, CA). polyI:C and LPS from Escherichia coli were from Sigma-Aldrich (St Louis, MO). rmGM-CSF, rmIL-4, and rmIL-33 were from Peprotech (Rochy Hill, NJ). Rabbit polyclonal IL-33 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal ST2 antibody was from Enzo (Farmigdale, NY). rmIL-33 and rmST2 soluble proteins were from R&D (Minneapolis, MN). Hamster monoclonal CD11c antibody was from Abcam (Cambrige, MA). Mouse IL-33 ELISA kit, purified anti-mouse NF-κB p65, purified anti-mouse CD16/32, fluorescein isothiocyanate–conjugated anti-mouse CD11c, allophycocyanin (APC)-conjugated anti-mouse CD40, APC-conjugated anti-mouse CD80, phycoerythrin(PE)-conjugated anti-CD86, and PE-conjugated anti-mouse I-A/I-E were from Biolegend (San Diego, CA). RNeasy Mini RNA extraction kit was from Qiagen (Valencia, CA); enhanced chemiluminescence reagents and Ready-To-Go-Primer First-Strand Beads were obtained from GE Healthcare (Piscataway, NJ); TaqMan gene expression assays and real-time PCR master mix were from Applied Biosystems (Foster City, CA); and horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G and BCA (bicinchoninic acid) protein assay kit were from Pierce Chemical (Rockford, IL).
Animal. The animal research protocol was approved by the Center for Comparative Medicine at Baylor College of Medicine. All animals used in this study were maintained in specific pathogen-free conditions in microisolator cages and were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. Female BALB/c mice at 6–8-weeks old were purchased from the Jackson Laboratory (Bar Harbor, ME). Heterozygous MyD88−/− mice on a C57BL/6 background were kindly provided by Dr Shizuo Akira (Research Institute for Microbial Disease, Osaka University, Japan) through Dr Eric Pearlman (Department of Ophthalmology and Visual Sciences, CaseWestern Reserve University, Cleveland, OH). The genotyping was performed by means of PCR of the tail DNA with three specific primers (MyD88F, TGGCATGCCTCCATCATAGTTAACC; MyD88R, GTCAGAAACAACCACCACCATGC; MyD88R-Neo, ATCGCCTTCTATCGCCTTCTTGACG) by using a previously described method.48 The MyD88−/− mice grown to 6–8 weeks were used for experiments.
Generation of murine bone marrow–derived Dcs. Bone marrow–derived DCs were generated as previously described22 with minor modifications. Briefly, 6–8-week-old female BALB/c mice were killed, femurs and tibiae were removed, and the marrow was flushed with RPMI-1640 using a syringe with a 0.3-mm needle. Clusters within the marrow suspension were disassociated by vigorous pipetting. Bone marrow cells were suspended in complete media (CM, RPMI-1640 supplemented with 10% fetal bovine serum, 50 μg ml−1 gentamicin, and 1.25 μg ml−1 amphotericin B). Cells were adjusted to 1.0 × 106 ml−1 and plated on 100-mm dish at 10 ml per well. They were cultured in CM containing15 ng ml−1 of rmGM-CSF and 5 ng ml−1 of rmIL-4 (CM-CSF-IL-4) at 37 °C and 5% CO2. On day 3 of culture, 10 ml of CM-CSF-IL-4 was added to the cells. On days 6 and 8, 10 ml of medium with cells were collected from each culture dish and centrifuged at 300 g for 5 min at room temperature; the cells were re-suspended in 10 ml of the same medium, and then given back to the dishes. On day 9, the non-adherent DCs were used for experiments.
Treatment of murine bone marrow-derived DCs. DCs at 1.0 × 106 per well in 12-well plates were incubated for 4–24 h with medium alone, TLR ligands (Pam3CSK4, PGN, polyI:C, LPS, flagellin, FSL-1, R837, ssRNA, or C-CpG-ODN, ligands to TLR 1–9 respectively, 10 μg ml−1 each, except for 50 μg ml−1 polyI:C and 1 μg ml−1 LPS), or IL-33 (1 ng ml−1) at the absence or presence of ST2 neutralizing antibody (5 μg ml−1) or soluble ST2 protein (5 μg ml−1) for mRNA expression. DCs at 1.0 × 106 or 5.0 × 106 were treated with LPS, flagellin, or IL-33 (1 ng ml−1) for 24 h in 500 μl medium for protein analysis by ELISA, immunofluorescent staining, western blotting, or flow cytometry.
A topical challenge murine model of ocular surface with LPS and flagellin. BALB/c mice were topically challenged in the conjunctival sac with 1 μg LPS or 1 μg flagellin in 5 μl PBS per eye three times a day for 2 days, 5 μl PBS alone was used as a control. Twenty-four hours after the final challenge, the whole globes and draining CLNs in each group (n=4) were excised, embedded in optimal cutting temperature compound (VWR, Suwanee, Ga), and flash-frozen in liquid nitrogen. Sagittal 8-mm cryosections from murine globes and CLNs were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and stored at −80°C before use.
A murine model of EAC induced by SRW pollen. The murine EAC model was induced using the previously reported methods.24, 25 In brief, mice were immunized with 50 μg of SRW pollen (Greer Laboratories, Lenoir, NC) in 5 mg of Imject Alum (Pierce Biotechnology) by footpad injection on day 0. Allergic conjunctivitis was induced by repeated topical challenges of 1.5 mg of SRW pollen suspended in 10 ml of PBS (pH 7.2) into each eye once a day from days 10–12. PBS eyedrop–treated SRW-sensitized and untreated mice were used as controls. On day 13, 24 h after the last SRW challenge, the whole eyeballs and CLNs were harvested for immunofluorescent staining.
RNA extraction, reverse transcription, and RT-qPCR. Total RNA was extracted with a RNeasy Micro Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, quantified with a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Wilmington, DE), and stored at −80°C. The first-strand cDNA was synthesized by reverse transcription from 1 μg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described.49, 50 Quantitative real-time PCR was performed (Mx3005P QPCR System; Stratagene, La Jolla, CA) with 20 μl reaction volume containing 5 μl of cDNA, 1 μl gene expression assay, and 10 μl gene expression master mix (TaqMan; Applied Biosystems, Foster City, CA). TaqMan gene expression assays were used: GAPDH (glyceraldehyde 3-phosphate dehydrogenase; Mm99999915_g1), IL-33 (Mm00505403_m1), MyD88 (Mm00440338_m1), NF-κB1 (Mm00476361_m1), NF-κB2 (Mm00479807_m1), RelA (Mm00501346_m1), CD40 (Mm00441891_m1), CD80 (Mm00711660_m1), OX40L (Mm00437214_m1), IL-4 (Mm00445259_m1), IL-5 (Mm99999063_m1), IL-13 (Mm00434204_m1), TNF-α (Mm99999068_m1), IL-1β (Mm 00434228_m1), and CCL17 (Mm00516136_m1). The thermocycler parameters were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A nontemplate control was included to evaluate DNA contamination. The results were analyzed by the comparative threshold cycle (Ct) method and normalized by GAPDH as an internal control.51
ELISA. Double-sandwich ELISA for mouse IL-33 was performed, according to the manufacturer’s protocol, to determine the IL-33 protein level in cell lysates with different treatment. Absorbance was read at a reference wavelength of 450 nm by a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
Western blot analysis. Western blot analysis was performed with a previously reported method.51 Briefly, the cell lysates (50 μg per lane, measured by a BCA protein assay kit) were mixed with 6 × SDS reducing sample buffer and boiled for 5 min before loading. The proteins were separated on an SDS polyacrylamide gel and electronically transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in TTBS (50 mM Tris (pH 7.5), 0.9% NaCl, and 0.1% Tween-20) for 1 h at room temperature and incubated, first with primary antibodies against IL-33 (1:100, 2 μg ml−1) or β-actin (1:500, 1 μg ml−1) overnight at 4 °C, and then with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The signals were detected with enhanced chemiluminescence reagent using a Kodak image station 2000R (Eastman Kodak, New Haven, CT).
Immunofluorescent staining. Indirect immunostaining was performed according to our previously reported methods.52, 53 In brief, DCs were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS at room temperature for 10 min, respectively, and frozen sections of the tissue were fixed with acetone at −30 °C for 5 min. Primary rabbit anti-mouse IL-33 (4 μg ml−1), rabbit anti-mouse NF-κB p65 (2 μg ml−1), and hamster anti-mouse CD11c (10 μg ml−1) were applied for 2 h. AlexaFluor 488-conjugated or 594-conjugated secondary antibodies (Invitrogen) were incubated for 1 h at room temperature, propidium iodide or 4′,6-diamidino-2-phenylindole were used for nuclear counterstaining. Secondary antibody alone or isotype immunoglobulin G were used as negative controls. The results were photographed with an epifluorescence microscope (Eclipse 400; Nikon, Garden City, NY) using a digital camera (DMX 1200, Nikon). For quantification, the positive cells were counted against total cells and represent as percentage (%) or per thousand (%o).54, 55, 56, 57
Flow cytometry analysis. Flow cytometry was performed as previously described22 with minor modifications. Briefly, DCs were blocked with anti-CD16/32 Ab (50 μg ml−1) for 10 min to block Fc receptors and were stained with fluorescein isothiocyanate–conjugated anti-CD11c (20 μg ml−1), APC-conjugated anti-CD40 (20 μg ml−1), APC-conjugated anti-CD80 (10 μg ml−1), PE-conjugated anti-CD86 (10 μg ml−1), PE-conjugated anti-I-A/I-E (10 μg ml−1), and PE-conjugated anti-MHC class II (10 μg ml−1) for 30 min at 4 °C. Dead cells were excluded from all analyses by staining with 10 μg ml−1 propidium iodide. The analysis was performed by the BD LSRII Benchtop cytometer by gating on a CD11c-positive forward scatter-high cell population, and data were analyzed using BD Diva Software (BD Pharmingen, San Jose, CA).
Statistical analysis. Student’s t-test was used to compare differences between the two groups. One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post-hoc test. P<0.05 were considered statistically significant.
References
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Smith, D.E. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin. Exp. Allergy 40, 200–208 (2010).
Prefontaine, D. et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 183, 5094–5103 (2009).
Matsuyama, Y. et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J. Rheumatol. 37, 18–25 (2010).
Cevikbas, F. & Steinhoff, M. IL-33: a novel danger signal system in atopic dermatitis. J Invest. Dermatol. 132, 1326–1329 (2012).
Brandt, E.B. & Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell Immunol. 2, 110 (2011).
Wisniewski, J.A. & Borish, L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 32, 83–94 (2011).
Kobori, A. et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 45, 999–1007 (2010).
Seidelin, J.B., Bjerrum, J.T., Coskun, M., Widjaya, B., Vainer, B. & Nielsen, O.H. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol. Lett. 128, 80–85 (2010).
Kamekura, R., Kojima, T., Takano, K., Go, M., Sawada, N. & Himi, T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin. Exp. Allergy 42, 218–228 (2012).
Matsuda, A. et al. The role of interleukin-33 in chronic allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 50, 4646–4652 (2009).
Matsuba-Kitamura, S. et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int. Immunol. 22, 479–489 (2010).
Moussion, C., Ortega, N. & Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3, e3331 (2008).
Luthi, A.U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Cayrol, C. & Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 106, 9021–9026 (2009).
Yanagawa, Y., Suzuki, M., Matsumoto, M. & Togashi, H. Prostaglandin E(2) enhances IL-33 production by dendritic cells. Immunol. Lett. 141, 55–60 (2011).
Yanagawa, Y., Matsumoto, M. & Togashi, H. Adrenoceptor-mediated enhancement of interleukin-33 production by dendritic cells. Brain Behav. Immun. 25, 1427–1433 (2011).
Alexopoulou, L. & Kontoyiannis, D. Contribution of microbial-associated molecules in innate mucosal responses. Cell Mol. Life Sci. 62, 1349–1358 (2005).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367 (2001).
Zhang, L., Lu, R., Zhao, G., Pflugfelder, S.C. & Li, D.Q. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int. J. Biochem. Cell Biol. 43, 1383–1391 (2011).
Williams, L.A., Egner, W. & Hart, D.N. Isolation and function of human dendritic cells. Int. Rev. Cytol. 153, 41–103 (1994).
Zheng, X., de Paiva, C.S., Li, D.Q., Farley, W.J. & Pflugfelder, S.C. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest. Ophthalmol. Vis. Sci. 51, 3083–3091 (2010).
Rank, M.A., Kobayashi, T., Kozaki, H., Bartemes, K.R., Squillace, D.L. & Kita, H. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 123, 1047–1054 (2009).
Zheng, X. et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 51, 3076–3082 (2010).
Li, D.Q. et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J. Allergy Clin. Immunol. 128, 1318–1325 (2011).
Johnson, A.C. et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest. Ophthalmol. Vis. Sci. 46, 589–595 (2005).
Piggott, D.A. et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J. Clin. Invest. 115, 459–467 (2005).
Idzko, M. et al. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J. Clin. Invest. 117, 464–472 (2007).
Besnard, A.G., Togbe, D., Guillou, N., Erard, F., Quesniaux, V. & Ryffel, B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur. J. Immunol. 41, 1675–1686 (2011).
Liu, X., Li, M., Wu, Y., Zhou, Y., Zeng, L. & Huang, T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 386, 181–185 (2009).
Moulin, D., Donze, O., Talabot-Ayer, D., Mezin, F., Palmer, G. & Gabay, C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 40, 216–225 (2007).
Smithgall, M.D., Comeau, M.R., Yoon, B.R., Kaufman, D., Armitage, R. & Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Cherry, W.B., Yoon, J., Bartemes, K.R., Iijima, K. & Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 (2008).
Suzukawa, M. et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab. Invest. 88, 1245–1253 (2008).
Wood, I.S., Wang, B. & Trayhurn, P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem. Biophys. Res. Commun. 384, 105–109 (2009).
Hammad, H., Chieppa, M., Perros, F., Willart, M.A., Germain, R.N. & Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Yang, M., Kumar, R.K. & Foster, P.S. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-gamma and TLR4/MyD88 pathways. J. Immunol. 182, 5107–5115 (2009).
Ortiz-Stern, A. et al. Langerin+ dendritic cells are responsible for LPS-induced reactivation of allergen-specific Th2 responses in postasthmatic mice. Mucosal Immunol. 4, 343–353 (2011).
Le, T.A. et al. Flagellin Induces the Expression of Thymic Stromal Lymphopoietin in Human Keratinocytes via Toll-Like Receptor 5. Int. Arch. Allergy Immunol. 155, 31–37 (2010).
Reginald, K. et al. Immunoglobulin E antibody reactivity to bacterial antigens in atopic dermatitis patients. Clin. Exp. Allergy 41, 357–369 (2011).
Prescott, S.L. et al. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J. Allergy Clin. Immunol. 122, 391–399 (2008).
Randolph, G.J., Angeli, V. & Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Allan, R.S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Medzhitov, R. & Janeway, C. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89–97 (2000).
Nolan, G.P., Ghosh, S., Liou, H.C., Tempst, P. & Baltimore, D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell 64, 961–969 (1991).
Song, X.J. et al. Neurturin-deficient mice develop dry eye and keratoconjunctivitis Sicca. Invest. Ophthalmol. Vis. Sci. 44, 4223–4229 (2003).
Luo, L., Li, D.Q., Doshi, A., Farley, W., Corrales, R.M. & Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. Ophthalmol. Vis. Sci. 45, 4293–4301 (2004).
Yoon, K.C. et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest. Ophthalmol. Vis. Sci. 48, 2561–2569 (2007).
Ma, P. et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest. Ophthalmol. Vis. Sci. 50, 2702–2709 (2009).
Kim, H.S., Jun Song, X., de Paiva, C.S., Chen, Z., Pflugfelder, S.C. & Li, D.-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 79, 41–49 (2004).
Chen, Z., de Paiva, C.S., Luo, L., Kretzer, F.L., Pflugfelder, S.C. & Li, D.-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22, 355–366 (2004).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998).
Jiang, W. et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375, 151–155 (1995).
Reche, P.A. et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167, 336–343 (2001).
Acknowledgements
This study was supported, in part, by the Department of Defense CDMRP PRMRP grant FY06 PR064719 (DQL), National Institutes of Health grant EY11915 (SCP), and an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Su, Z., Lin, J., Lu, F. et al. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol 6, 921–930 (2013). https://doi.org/10.1038/mi.2012.130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.130
This article is cited by
-
Linalyl Acetate Ameliorates Mechanical Hyperalgesia Through Suppressing Inflammation by TSLP/IL-33 Signaling
Neurochemical Research (2022)
-
Egg yolk augments type 2 immunity by activating innate cells
European Journal of Nutrition (2020)
-
Short ragweed pollen promotes M2 macrophage polarization via TSLP/TSLPR/OX40L signaling in allergic inflammation
Mucosal Immunology (2019)
-
IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection
Nature Communications (2019)
-
Oxidative stress enhances the expression of IL-33 in human airway epithelial cells
Respiratory Research (2018)