Abstract
In soil, the way biotic parameters impact the relationship between bacterial diversity and function is still unknown. To understand these interactions better, we used RNA-based stable-isotope probing to study the diversity of active atrazine-degrading bacteria in relation to atrazine degradation and to explore the impact of earthworm-soil engineering with respect to this relationship. Bulk soil, burrow linings and earthworm casts were incubated with 13C-atrazine. The pollutant degradation was quantified by liquid chromatography–mass spectrometry for 8 days, whereas active atrazine degraders were identified at 2 and 8 days by sequencing the 16S ribosomal RNA in the 13C-RNA fractions from the three soil microsites. An original diversity of atrazine degraders was found. Earthworm soil engineering greatly modified the taxonomic composition of atrazine degraders with dominance of α-, β- and γ-proteobacteria in burrow linings and of Actinobacteria in casts. Earthworm soil bioturbation increased the γ-diversity of atrazine degraders over the soil microsites generated. Atrazine degradation was enhanced in burrow linings in which primary atrazine degraders, closely related to Pelomonas aquatica, were detected only 2 days after atrazine addition. Atrazine degradation efficiency was not linearly related to the species richness of degraders but likely relied on keystone species. By enhancing soil heterogeneity, earthworms sustained high phylogenetic bacterial diversity and exerted a biotic control on the bacterial diversity–function relationships. Our findings call for future investigations to assess the ecological significance of biotic controls on the relationships between diversity and function on ecosystem properties and services (for example, soil detoxification) at larger scales.
Similar content being viewed by others
Introduction
Ecological theories have mainly been developed to address questions about macroorganisms. However, given that microorganisms play a significant role in virtually every ecosystem, the extent to which these theories apply to the microbial world is of concern. Microorganisms should be used to test general ecological theories in better-controlled and more manipulable experimental systems (Prosser et al., 2007). Such experiments could prove existing theories, test whether they can be generalised between unicellular and multicellular organisms and could provide new findings of wide importance in ecology and evolution at various time and spatial scales (Beaumont et al., 2009).
In soil ecosystems, a better understanding of microbial community structure, function and biotic and abiotic interactions is of interest, as microorganisms play a critical role in biogeochemical cycling and are drivers of soil ecosystem functioning and services. Soil microorganisms take part in ∼90% of the processes occurring in soil (Nannipieri et al., 2003). This dependency of terrestrial ecosystem functioning on microorganisms implies that changes in microbial diversity should have a significant impact on the ecosystem performance. Different relationships between diversity and ecosystem function have been proposed so far (Peterson et al., 1998). With regard to soil microorganisms, previous studies have shown that the activity of different functional groups is not affected by a decline in soil microbial diversity (Griffiths et al., 2001; Wertz et al., 2006, 2007). These results indicate high stability of the studied soil systems and suggest the existence of functional redundancy among soil microorganisms, leading to ecosystem resistance and resilience (Walker, 1995). However, functional redundancy is likely more limited within specialised rather than global processes (Swift et al., 2004). At a local scale, in situations where general and specialised ecological functions are taken into account, and where the environment is changing because of biotic and abiotic factors, other ecological theories such as the rivet hypothesis may be more appropriate. The rivet hypothesis predicts less strongly saturating associations between diversity and ecological function (Ehrlich and Ehrlich, 1981). A way to test the applicability of these diversity–function relationship theories to microbial ecology is to identify the microorganisms actively involved in a particular function in connection with different environmental parameters. This can now be addressed thanks to the recent development of new molecular tools such as stable-isotope probing (SIP) of environmental nucleic acids (DNA or RNA) (Radajewski et al., 2000). RNA-based SIP has thus been used to study different degrading microorganisms in different environments or to determine the selective impact of organic molecule addition on active bacterial communities in soil (for example, Manefield et al., 2002; Monard et al., 2008a; Vandenkoornhuyse et al., 2007).
In soil, bacterial communities are closely shaped by the biological reworking of the soil matrix performed by inhabiting macroorganisms, such as plant roots or macrofauna (Jones et al., 1997; Meysman et al., 2006). By engineering the soil, earthworms generate various soil microsites that differ from the bulk soil in terms of microporosity, moisture, nutrient content or oxygenation (Pivetz and Steenhuis, 1995; Devliegher and Verstraete, 1997; Decaëns et al., 1999; Blanchart et al., 2004; Le Bayon and Binet, 2006). Earthworms thus cause, in part, the intrinsic soil heterogeneity that is known to be an important factor influencing microbial abundance, diversity and activity as proposed by the niche-based microbial response theory (Furlong et al., 2002; Ramette and Tiedje, 2007; Monard et al., 2008b). It can therefore be hypothesised that soil bioturbation resulting from earthworm activity may significantly improve soil functioning by increasing the γ-diversity within functional groups and the associated functional redundancy.
In this study, RNA-based SIP was used to test the hypothesis that changes occurring in soil bacterial diversity due to biotic interactions, such as earthworm bioturbation, induce significant modifications in soil functioning. We focused on the pollutant degradation service sustained by soil, and atrazine was chosen as model organic molecule. It has been shown that atrazine biodegradation can be carried out by individual strains of bacteria, but catabolic pathways involving bacterial consortia are likely to be more common in soils (de Souza et al., 1998a; Smith et al., 2005; Chirnside et al., 2007; Kolic et al., 2007). We thus analysed the relationships between active atrazine degrader diversity and the specific atrazine degradation function under biotic control generated by earthworms. RNA-based SIP was used by applying 13C-labelled atrazine to bulk soil and soil engineered by earthworms (casts and burrow linings). The active atrazine degrader diversity was determined after 2 and 8 days following atrazine soil treatment by analysing the 16S ribosomal RNA sequences in 13C-labelled RNA. Liquid chromatography–mass spectrometry was simultaneously performed to measure 13C-atrazine degradation in the samples. We demonstrated that earthworm soil engineering, by enhancing soil heterogeneity, increased the soil bacterial γ-diversity as well as a high atrazine-degrading bacterial diversity, and thus exerted a biotic control on the bacterial diversity–function relationship. Atrazine degradation efficiency was shown not to be linearly related to the species richness of degraders but relied mostly on keystone species pertaining to a larger consortium.
Materials and methods
Soil microcosms
Soil was collected from the 0–30 cm top horizon layer at the agricultural site of the Institut National de Recherche Agronomique (Epoisses, France). This soil was a clay loam (sand 6.7%, silt 47.2% and clay 46.1%) and had a pH of 6.7. The collected fresh soil was sieved at 4 mm and stored at 4 °C until use. Soil microcosms were prepared as described by Binet and Trehen (1992). Each microcosm (PVC cylinder; 15 cm high and 12 cm diameter) was filled with 2280 g of 4 mm-sieved fresh soil (water content of 22% w/w). The soil in each microcosm was compacted to a bulk density of 1.46 g cm−3. The microcosms were placed in a climate chamber at 15 °C under a 12:12 h night/day photoperiod. After 1 day, three adult specimens of the earthworm species Lumbricus terrestris were added to the soil surface with a total average biomass of 8.2±0.3 g per microcosm. Every 2 days, earthworm surface casts were harvested and stored at −20 °C until use. After 20 days of earthworm addition, soil microcosms were destroyed for sampling. The bulk soil not burrowed by earthworms and the burrow linings, corresponding to the 1 mm thickness of soil carefully collected with a fine spatula from the sides of the entire burrow network, were sampled and stored at −20 °C until use for atrazine treatment and SIP analysis. It is likely that freezing can cause biases in bacterial diversity, but the biases, if they exist, are expected to be of the same range throughout the samples. In addition, this storage strategy has been shown to provide a good representation of the initial state (Stenberg et al., 1998).
Soil microsite atrazine treatments
The water content of the three soil microsites (bulk soil, burrow linings and casts) was adjusted to 0.5 g water per g dry soil (105 °C). Subsequently, 12 g of each moistened soil microsite were treated with 25 mg of atrazine (chemical purity 98%, Syngenta Crop Protection, Basel, Switzerland) per g of dry soil. The soil samples were then placed in a sterile glass jar and preincubated at 20 °C in the dark for 20 days.
Each pretreated sample was then treated with 25 mg of 13C-chain-labelled atrazine (chemical purity 97%, isotopic enrichment 98%, Innovation & Chimie Fine, Manosque, France) per g of dry soil and incubated at 20 °C in the dark. 13C-atrazine degradation was monitored for 8 days, whereas RNA was extracted after 2 and 8 days following atrazine treatment. Additional control experiments with unlabelled atrazine were performed on bulk soil to ensure the absence of 13C-RNA in heavy fraction of the density gradient.
Degradation monitoring of 13C-atrazine and metabolites
To monitor atrazine degradation, 13C-atrazine along with its metabolites (13C-hydroxyatrazine, 13C-deethylatrazine and 13C-deisopropylatrazine) were measured in the soil microsites for the time course of the incubation by liquid chromatography–mass spectrometry (Waters alliance 2690, Waters, Saint Quentin en Yvelines, France). To do so, 250 mg of soil sample were dissolved in 1 l of mineral water. After 15 min of ultrasound treatment, the sample was mixed during 1 h and subjected to 15 min ultrasound treatment again. Subsamples of 50 ml of the mixture were diluted 10 times, shaken during 15 min and 5 ml were collected and mixed with 10 μl of a methoprothryne solution (20 mg l−1). Atrazine and its metabolites were then selectively extracted by solid phase extraction with Oasis hydrophilic—lipophilic balance extraction cartridges (200 mg, 6 ml, Waters), preconditioned with 6 ml CH2Cl2/CH3CN (60/40) and 6 ml MeOH, followed by 6 ml of ultrapure water. Two elutions with 5 ml of CH2Cl2/CH3CN (60/40) were performed and concentrated to 1 ml by gentle nitrogen stream evaporation. To avoid extract to dry up, evaporation was performed to a final volume of 10 μl, subsequently adjusted to 1 ml with H2O/CH3CN (90/10) acidified (0.1% formic acid). This sample was analysed by liquid chromatography–mass spectrometry. Atrazine and metabolites were separated on a column X Terra MS C18 (Waters, Saint Quentin en Yvelines, France); 150 × 2.1 mm, 3.5 μm particle size at 35 °C. A binary mobile phase gradient (ultrapure water with 0.1% formic acid and CH3CN with 0.1% formic acid) was used for pesticide separation. A simple quadrupole mass spectrometer model ZQ (Waters-Micromass, Saint Quentin en Yvelines, France) equipped with an electrospray source coupled with high-performance liquid chromatography was employed. For ion monitoring, the cone voltage was optimised by continuous infusion to achieve the highest sensitivity possible.
Soil RNA extraction
RNA was extracted from 250 mg of fresh soil after 2 and 8 days of incubation according to Monard et al. (2010). The RNA pellets were washed in 75% ethanol, dried and resuspended in 50 μl of ultrapure DNase- and RNase-free water. RNA was quantified at 260 nm, and RNA purity was estimated by calculating the ratio between the absorbance at 260 and 280 nm (NanoDrop Technologies, Wilmington, DE, USA). RNAs were aliquoted and stored at −80 °C.
Density gradient centrifugation and fractionation
Isopycnic centrifugation was performed with 50 ng purified RNA according to Vandenkoornhuyse et al. (2007). Representative fractions of 12C-(light density fraction, 1.77–1.79 g ml−1) and 13C-labeled (heavy density fraction, 1.83–1.85 g ml−1) RNAs, in agreement with Whiteley et al. (2007) and Vandenkoornhuyse et al. (2007), were carefully taken along the ultracentrifugation gradient using syringe and needles. The position of the two fractions along the density gradient was determined by previous bacterial culture experiments with 12C- and 13C-labeled glucose (Supplementary Information). Furthermore, unlabelled control samples were used to check the absence of RNA within the heavy fractions (see reverse transcriptase-PCR section below). Each RNA fraction was precipitated with two volumes of isopropanol. After centrifugation, the pellets were washed in 75% ethanol, dried and redissolved in 25 μl ultrapure water.
Reverse transcriptase-PCR
From 12C-RNA and 13C-RNA templates, reverse transcriptase-PCRs were performed from an aliquot of 4 μl of RNA using the Titan One Tube RT-PCR Kit (Roche Applied Science, Mannheim, Germany). The reaction was carried out in a final volume of 50 μl with 10 pmol of the primers Eub_519f and Eub_1390r (Eurofins MWG Operon, Ebersberg, Germany) specific for bacteria (modified in Orphan et al., 2000; Vandenkoornhuyse et al., 2007). Similarly to our previous studies (Vandenkoornhuyse et al., 2007; Monard et al., 2008a), no amplified fragments were detected in the heavy density fraction collected from the control experiment with unlabelled RNA.
Cloning and sequencing
The amplified fragments were ligated in the pGEM-T vector (Promega, Madison, WI, USA), and DH5α Library efficiency competent cells (Invitrogen, Carlsbad, CA, USA) were transformed by heat shock. Cells were grown in Luria–Bertani medium at 37 °C overnight. The culture medium was supplemented with ampicillin (125 mg l−1), isopropyl β-D-1-thiogalactopyranoside (0.1 mM) and X-Gal (20 mg l−1). From each clone library, both strands of 135- to 219-positive clones were sequenced (Genoscreen, Lille, France) using the T7 and SP6 primers. The two sequences were aligned, and each nucleotide position was checked using Sequencher 4.6 (GeneCodes, Ann Arbor, MI, USA). Sequences were searched for possible chimeric sequences (Chimera Check, http://www.mothur.org/). The sequences were submitted to the GenBank database under accession numbers GU290552–GU291238.
Diversity and phylogenetic analyses
α- and γ-Diversities were estimated using Shannon's information measure H0 for each and for all soil microsites, respectively. The specific richness S was expressed as the number of phylotypes, and the β-diversity was determined as the Sørensen's similarity index between earthworm soil microsites (burrow linings and casts) and bulk soil.
Rarefaction curves were computed for each data set (Supplementary Figure S1). The number of species was quantified for 100 random combination of 1 to N sequences and also by performing 100 bootstrap pseudoreplicates implemented in estimates (Colwell et al., 2004). Multiple alignments were implemented with CLUSTALX (Thompson et al., 1997) and refined by hand. Analyses of influence function were performed to remove outlying nucleotides from the matrices according to Bar-Hen et al. (2008). PAUP 4.0 β 10 (Sinauer Associates, Sunderland, MA, USA) was used to compute the maximum parsimony trees by using a heuristic tree search with 500 replicates of random addition, tree bisection and reconnection as swapping algorithm. For the 1000 bootstrap pseudoreplicates, 15 replicates of random addition using the tree bisection and reconnection swapping procedure were performed. Along with these phylogenetic reconstructions, the maximum likelihood tree was calculated using PAUP 4.0 β 10 using the GTR+I+G model with bootstrap estimates from 100 iterations. Modeltest 3.7 software was used to select the model (Posada and Crandall, 1998). Finally, neighbour-joining reconstructions were also computed using K2P model to check in depth the congruence among trees. An uncultured crenarchaeote (AJ347774) was used as outgroup (Supplementary Figures S2, S3 and S4).
Results and discussion
Biotic control of atrazine degradation function
13C-atrazine degradation was faster in burrow linings than in bulk soil and casts (Figure 1). After 8 days, 48%, 64% and 73% of the initial atrazine added were measured in burrow linings, bulk soil and casts, respectively (Figure 1). The associated final degradation rates were 2.1, 0.4 and 0.9 mg of atrazine per g per day, respectively. Thus, 8 days after atrazine treatment, atrazine degradation was still more efficient in burrow linings compared with the other soil microsites. Whereas hydroxyatrazine and deethylatrazine were detected at low concentrations in all soil microsites (at most 0.25 mg and 0.17 mg per g dry soil, respectively, data not shown), deisopropylatrazine was not detected. Thus, as already observed (Kersante et al., 2006; Monard et al., 2008b), earthworm soil bioturbation impacted atrazine mineralisation kinetics, and our hypothesis is that this should be mainly explained by changes in the diversity of atrazine degraders in the engineered soil microsites.
13C-labeled atrazine degradation kinetics in bulk soil (straight line), burrow linings (dash line) and earthworm casts (dot line). Error bars indicate s.d. Star represents statistical significant differences (P<0.001) between the atrazine degradation kinetics of the soil microsites determined by analysis of variance using Minitab software (State College, PA, USA).
Biotic control of atrazine degrader diversity
After 2 days of 13C-atrazine treatment, active atrazine degraders were only detected in burrow linings and corresponded to a unique phylotype highly similar to the strain Pelomonas aquatica (Figure 2 and Supplementary Figure S2A). This β-proteobacteria isolated from industrial water in Sweden (Gomila et al., 2007) has not been described as an atrazine degrader before this.
Phylogenetic affinities of bacterial small-subunit (SSU) ribosomal RNA sequences recovered from 13C-RNA in burrow linings at 2 days following atrazine treatment (purple) and from bulk soil (blue), burrow linings (red) and casts (green) at 8 days following atrazine treatment. The tree was computed by NJ using K2P model (scale bar: 0.1 substitution per site) of all the bacterial sequences amplified, with representatives of the highest BLAST hit of each phylotype and known atrazine degraders in orange.
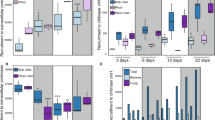
After 8 days of 13C-atrazine treatment, active atrazine degraders were detected in all soil microsites and their taxonomic composition varied greatly between the different soil samples (Figures 2 and 3). The atrazine degraders in bulk soil were dominated by the Actinobacteria and the β-proteobacteria (46.2% and 28.6% of clones, respectively) (Figures 2, 3 and Supplementary Figure S3). Similar bacterial groups involved in atrazine degradation were detected in burrow linings, except for the notable absence of Chloroflexi. However, there were significant differences in the relative abundance of bacterial groups, and the communities were dominated by α-, β- and γ-proteobacteria (31.4%, 40.7% and 17.4% of clones, respectively) (Figures 2, 3 and Supplementary Figure S2B). In casts, the atrazine degraders were greatly dominated by the Actinobacteria corresponding to a single phylotype closely related to Micrococcus luteus (72.5% of clones) (Figures 2, 3 and Supplementary Figure S4). The loss of diversity in casts is consistent with the negative impact of earthworm soil digestion on the number of soil bacteria that has been recently observed by culture-independent methods (Monard et al., 2008b). Earthworm bioturbation impacted active atrazine degrader diversity by modifying taxonomic group dominance (species evenness) and composition (species richness S) of these degraders (Figure 3). Few previous studies have investigated the total bacterial diversity in soil microsites generated by earthworms. With both culture-dependant and culture-independent techniques, changes in total bacterial community diversity in burrow linings and casts relative to bulk soil have already been observed; the importance of particular bacterial groups being enhanced and that of others reduced in the different soil microsites depending on the initial soil and earthworm food resources (Furlong et al., 2002; Schonholzer et al., 2002; Tiunov and Dobrovolskaya, 2002; Egert et al., 2004). In the present study, by analysing a specific function (that is, atrazine degradation), we demonstrated that earthworm bioturbation, by generating heterogeneity in soil, contributes to the maintenance of high atrazine degrader diversity, as some bacterial phylotypes were specific to soil microsites (the β-similarity index was low, ranging from 0.24–0.33 between earthworm soil microsites and bulk soil; Figure 3). Even if the α-diversity of the atrazine degraders was lower within the burrow linings and casts (H0=2.2 and 1, respectively), earthworm bioturbation enhanced the γ-diversity over the soil microsites they generated (H0=2.7) (Figure 3). A total of 45% of the total species richness was specific to earthworm soil microsites (Figure 3). By acting on total soil bacterial diversity, earthworm bioturbation also modified specific functional diversity, such as atrazine-degrading bacteria as shown herein or methanotrophic bacteria as recently observed by Hery et al. (2007).
Abundance of the different bacterial phylogenetic groups retrieved in 13C-RNA (active atrazine degraders) and in both 12C- and 13C-RNA (total active bacteria) expressed for each (α-diversity) and for all soil microsites (γ-diversity). The specific richness S is expressed as the number of phylotypes, the diversity corresponds to the Shannon's information measure H0 and the β-diversity is determined as the Sørensen's similarity index between earthworm soil microsites (burrow linings and casts) and bulk soil.
Atrazine degrader diversity
The present paper shows that the atrazine degradation function was sustained by numerous different bacterial phylotypes, among which only two were already known as atrazine degraders (Ralstonia basilensis M91-3 and Pseudomonas sp. ADP, Mandelbaum et al., 1995; Stamper et al., 2002) (Figures 2, Supplementary Figures S2, S3 and S4). The high diversity of active atrazine degraders, we report herein, may be explained by horizontal transfers of genes involved in atrazine degradation (Tam et al., 1987; de Souza et al., 1998b; Top and Springael, 2003). Compared with other organisms, the existence of horizontal gene transfer in bacteria is important to consider when focusing on the relationship between diversity and function, and must be taken into account when referring to existing macroecological theories. The accessory genome that is acquired in part by gene transfer and encodes specific ecological adaptation or function is independent of the core genome used to define the bacterial diversity (Prosser et al., 2007), and horizontal gene transfer should induce the possibility of added function within the ecosystem, even with an apparent constant diversity (Vandenkoornhuyse et al., 2010). Thus, we cannot consider a functional trait as a proxy of species in microorganisms, and it can be concluded that the rivet hypothesis is a better fit for the bacterial world than other theories linking diversity and function.
Bacterial diversity–function relationship and earthworm biotic control
Consistent with faster and sustainable atrazine degradation (Figure 1; F=18.26, P<0.001), active atrazine degraders were rapidly detected in burrow linings after 2 days of incubation (Figures 2 and Supplementary Figure S2A). The corresponding phylotype, highly similar to P. aquatica, seemed to play a major role in atrazine degradation in this soil microsite. However, its presence alone might not be sufficient to enhance atrazine degradation, as it was detected in casts in which no enhanced degradation was observed (Figures 1 and 2). The enhanced degradation in burrow linings might be a result of a consortium with a particular community composition, optimal for atrazine degradation and absent or not fulfilled in casts.
By comparing the atrazine degradation kinetics (Figure 1) and the number of atrazine degrader phylotypes retrieved after 8 days of incubation in the three soil microsites (Figures 2, Supplementary Figures S2B, S3 and S4), no apparent linear relationship was observed between species richness and degradation function, despite that the sequencing depth was high enough to describe the diversity regardless the sample analysed (Supplementary Figure S1). Because atrazine degradation does not seem to depend on total atrazine degrader diversity, we can argue the existence of key atrazine degraders acting in a consortium. More than the quantitative diversity (the number of different degrading phylotypes), the qualitative diversity (identity and association of degraders) might be important to explain the changes in degradation kinetics. The highest yield of atrazine degradation observed in burrow linings was not associated with an increase in atrazine degrader diversity compared with the bulk soil. This cannot be explained by a better availability of the pesticide in burrow–linings, as atrazine supply was not limitative in all samples but this strongly suggests that the atrazine degraders are either more abundant or more active in this soil microsite. Previous studies have reported a concentration of atzA gene, the first key gene involved in atrazine degradation, in burrow linings as well as modification of atrazine-degrading gene expression after soil bioturbation by earthworms (Monard et al., 2008b, 2010). Considering all these elements, our study supports Walker's hypothesis that assumes coexistence of ‘drivers’ and ‘passengers’ referring to strong and low involvements in a given function, respectively (Walker, 1992). Thus, higher efficient atrazine degraders, the ‘drivers’, should have been selected in burrow linings.
According to the insurance hypothesis (Yachi and Loreau, 1999), high diversity may not enhance function over short term, but it may provide higher stability in the system over longer time periods. The apparent functional redundancy over small scales observed in this study may not be detected when the environment changes over larger spatial and time scales, as the species involved may be functionally differentiated (Rosenfeld, 2002). The atrazine degrader diversity in the burrow linings evolved from 2 to 8 days of incubation, and the individual fitness of these bacteria should be expected to change in this changing environment.
As the link between atrazine degrader diversity and the atrazine degradation function differs among the different soil microsites, earthworm soil bioturbation exerts a biotic control on this relationship. This control might be mostly generated through modification of soil physicochemical properties induced by earthworms that leads to the formation of various ecological niches for soil bacteria (Lavelle, 1988). Soil bacteria adapted to the local abiotic conditions at the micrometric scale within the microsite are not necessarily the most efficient at atrazine degradation. However, in the burrow linings that constitute an enriched microhabitat with higher organic matter, inorganic nitrogen and phosphorus, organic and total carbon (Parkin and Berry, 1999; Tiunov and Scheu, 1999; Jegou et al., 2001; Tiunov et al., 2001; Le Bayon and Binet, 2006), and also an oxic soil hot spot, the physicochemical properties should be favourable for efficient atrazine degraders as already suggested by Monard et al. (2008b). This increased atrazine-degrading activity might depend upon the presence of both efficient consortia and individual strains able to completely degrade atrazine such as Pseudomonas sp. ADP (Mandelbaum et al., 1995) whose related phylotype has only been detected in burrow linings (Figure 2). Thus, soil spatial heterogeneity, as the one generated by earthworm bioturbation, is an important process in sustaining bacterial diversity as well as functional diversity. In this study, we show that keystone species in atrazine degradation were selected in specific microhabitats. We should therefore argue in a wider vision that we have to consider the soil as a mosaic of different biota. Future research programmes analysing soil metagenomics, for example, must consider this fact with care.
Conclusion
The present study brings new insight into microbial ecology and the relationship between bacterial diversity and the function it sustains. It is the first report on the biotic control of this link. Thanks to the RNA-based SIP technique, we identified specific soil bacteria actively involved in the atrazine degradation process and demonstrated that the function is performed by a variety of phylogenetically different bacteria. More than the quantitative diversity corresponding to the number of different phylotypes involved, we concluded that the qualitative diversity of bacterial consortia and thus keystone phylotypes are important to maintain function. Earthworm soil bioturbation, by creating soil heterogeneity, enhanced the γ-diversity of bacteria, impacted the efficiency of the function studied and modified the diversity–function relationship among the soil microsites they generated. Future investigations are required to assess the ecological significance of such biotic control of the diversity–function relationship on ecosystem properties as well as on ecosystem services (such as soil detoxification) at larger scales. With regard to the microbial intrinsic abilities of short time generation, high-frequency gene transfer or high versatility between active and dormant stages, our results suggest that applicability of ecological theories to microbial ecology requires specific attention and new theoretical ideas.
Accession codes
References
Bar-Hen A, Mariadassou M, Poursat MA, Vandenkoornhuyse P . (2008). Influence function for robust phylogenetic reconstructions. Mol Biol Evol 25: 869–873.
Beaumont HJE, Gallie J, Kost C, Ferguson GC, Rainey PB . (2009). Experimental evolution of bet hedging. Nature 462: 90–93.
Binet F, Trehen P . (1992). Experimental microcosm study of the role of Lumbricus terrestris (oligochaeta:lumbricidae) on nitrogen dynamics in cultivated soils. Soil Biol Biochem 24: 1501–1506.
Blanchart E, Albrecht A, Brown G, Decaens T, Duboisset A, Lavelle P et al. (2004). Effects of tropical endogeic earthworms on soil erosion. Agric Ecosyst Environ 104: 303–315.
Chirnside AEM, Ritter WF, Radosevich M . (2007). Isolation of a selected microbial consortium from a pesticide-contaminated mix-load site soil capable of degrading the herbicides atrazine and alachlor. Soil Biol Biochem 39: 3056–3065.
Colwell RK, Mao CX, Chang J . (2004). Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85: 2717–2727.
de Souza ML, Newcombe D, Alvey S, Crowley DE, Hay A, Sadowsky MJ et al. (1998a). Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. Appl Environ Microbiol 64: 178–184.
de Souza ML, Wackett LP, Sadowsky MJ . (1998b). The atzABC genes encoding atrazine catabolism are located on a self-transmissible plasmid in Pseudomonas sp. strain ADP. Appl Environ Microbiol 64: 2323–2326.
Decaëns T, Rangel AF, Asakawa N, Thomas RJ . (1999). Carbon and nitrogen dynamics in ageing earthworm casts in grasslands of the eastern plains of Colombia. Biol Fertil Soils 30: 20–28.
Devliegher W, Verstraete W . (1997). Microorganisms and soil physico-chemical conditions in the drilosphere of Lumbricus terrestris. Soil Biol Biochem 29: 1721–1729.
Egert M, Marhan S, Wagner B, Scheu S, Friedrich MW . (2004). Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol Ecol 48: 187–197.
Ehrlich PR, Ehrlich AH . (1981). Extinction. The causes and Consequences of the Disapearance of Species. Random House: New York.
Furlong MA, Singleton DR, Coleman DC, Whitman WB . (2002). Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl Environ Microbiol 68: 1265–1279.
Gomila M, Bowien B, Falsen E, Moore ERB, Lalucat J . (2007). Description of Pelomonas aquatica sp. nov. and Pelomonas puraquae sp. nov., isolated from industrial and haemodialysis water. Int J Syst Evol Microbiol 57: 2629–2635.
Griffiths BS, Ritz K, Wheatley R, Kuan HL, Boag B, Christensen S et al. (2001). An examination of the biodiversity-ecosystem function relationship in arable soil microbial communities. Soil Biol Biochem 33: 1713–1722.
Hery M, Singer AC, Kumaresan D, Bodrossy L, Stralis-Pavese N, Prosser JI et al. (2007). Effect of earthworms on the community structure of active methanotrophic bacteria in a landfill cover soil. ISME J 2: 92–104.
Jegou D, Schrader S, Diestel H, Cluzeau D . (2001). Morphological, physical and biochemical characteristics of burrow walls formed by earthworms. Appl Soil Ecol 17: 165–174.
Jones CG, Lawton JH, Shachak M . (1997). Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78: 1946.
Kersante A, Martin-Laurent F, Soulas G, Binet F . (2006). Interactions of earthworms with atrazine-degrading bacteria in an agricultural soil. FEMS Microbiol Ecol 57: 192–205.
Kolic NU, Hrsak D, Begonja Kolar A, Petric I, Stipicevic S, Soulas G et al. (2007). Combined metabolic activity within an atrazine-mineralizing community enriched from agrochemical factory soil. Int Biodet Biodeg 60: 299–307.
Lavelle P . (1988). Earthworm activities and the soil system. Biol Fertil Soils 6: 237–251.
Le Bayon RC, Binet F . (2006). Earthworms change the distribution and availability of phosphorous in organic substrates. Soil Biol Biochem 38: 235–246.
Mandelbaum RT, Allan DL, Wackett LP . (1995). Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol 61: 1451–1457.
Manefield M, Whiteley AS, Griffiths RI, Bailey MJ . (2002). RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl Environ Microbiol 68: 5367–5373.
Meysman FJR, Middelburg JJ, Heip CHR . (2006). Bioturbation: a fresh look at Darwin's last idea. Trends Ecol Evol 21: 688–695.
Monard C, Binet F, Vandenkoornhuyse P . (2008a). Short-term response of soil bacteria to carbon enrichment in different soil microsites. Appl Environ Microbiol 74: 5589–5592.
Monard C, Martin-Laurent F, Devers-Lamrani M, Lima O, Vandenkoornhuyse P, Binet F . (2010). atz Gene expressions during atrazine degradation in the soil drilosphere. Mol Ecol 19: 749–759.
Monard C, Martin-Laurent F, Vecchiato C, Francez A-J, Vandenkoornhuyse P, Binet F . (2008b). Combined effect of bioaugmentation and bioturbation on atrazine degradation in soil. Soil Biol Biochem 40: 2253–2259.
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G . (2003). Microbial diversity and soil functions. Eur J Soil Science 54: 655–670.
Orphan VJ, Taylor LT, Hafenbradl D, Delong EF . (2000). Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl Environ Microbiol 66: 700–711.
Parkin TB, Berry EC . (1999). Microbial nitrogen transformations in earthworm burrows. Soil Biol Biochem 31: 1765–1771.
Peterson G, Allen CR, Holling CS . (1998). Ecological resilience, biodiversity, and scale. Ecosystems 1: 6–18.
Pivetz BE, Steenhuis TS . (1995). Soil matrix and macropore biodegradation of 2,4-D. J Environ Qual 24: 564–570.
Posada D, Crandall KA . (1998). MODELTEST: testing the model of DNA substitution. Bioinform Appl Note 14: 817–818.
Prosser JI, Bohannan BJM, Curtis TP, Ellis RJ, Firestone MK, Freckleton RP et al. (2007). The role of ecological theory in microbial ecology. Nat Rev Microbiol 5: 384–392.
Radajewski S, Ineson P, Parekh NR, Murrell JC . (2000). Stable-isotope probing as a tool in microbial ecology. Nature 403: 646–649.
Ramette A, Tiedje JM . (2007). Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc Natl Acad Sci USA 104: 2761–2766.
Rosenfeld JS . (2002). Functional redundancy in ecology and conservation. Oikos 98: 156–162.
Schonholzer F, Hahn D, Zarda B, Zeyer J . (2002). Automated image analysis and in situ hybridization as tools to study bacterial populations in food resources, gut and cast of Lumbricus terrestris L. J Microbiol Methods 48: 53–68.
Smith D, Alvey S, Crowley DE . (2005). Cooperative catabolic pathways within an atrazine-degrading enrichment culture isolated from soil. FEMS Microbiol Ecol 53: 265–275.
Stamper DM, Radosevich M, Hallberg KB, Traina SJ, Tuovinen OH . (2002). Ralstonia basilensis M91-3, a denitrifying soil bacterium capable of using s-triazines as nitrogen sources. Can J Microbiol 48: 1089.
Stenberg B, Johansson M, Pell M, Sjödahl-Svensson K, Stenström J, Torstensson L . (1998). Microbial biomass and activities in soil as affected by frozen and cold storage. Soil Biol Biochem 30: 393–402.
Swift MJ, Izac AMN, van Noordwijk M . (2004). Biodiversity and ecosystem services in agricultural landscapes—are we asking the right questions? Agric Ecosyst Environ 104: 113–134.
Tam AC, Behki RM, Khan SU . (1987). Isolation and characterization of an s-ethyl-N,N-dipropylthiocarbamate-degrading Arthrobacter strain and evidence for plasmid-associated s-ethyl-N,N-dipropylthiocarbamate degradation. Appl Environ Microbiol 53: 1088–1093.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG . (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882.
Tiunov AV, Bonkowski M, Bonkowski M, Tiunov JA, Scheu S . (2001). Microflora, Protozoa and Nematoda in Lumbricus terrestris burrow walls: a laboratory experiment. Pedobiologia 45: 46–60.
Tiunov AV, Dobrovolskaya TG . (2002). Fungal and bacterial communities in Lumbricus terrestris burrow walls: a laboratory experiment. Pedobiologia 46: 595–605.
Tiunov AV, Scheu S . (1999). Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol Biochem 31: 2039–2048.
Top EM, Springael D . (2003). The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr Opin Biotechnol 14: 262–269.
Vandenkoornhuyse P, Dufresne A, Quaiser A, Gouesbet G, Binet F, Francez A-J et al. (2010). Integration of molecular functions at the ecosystemic level: breakthroughs and future goals of environmental genomics and post-genomics. Ecol Lett 13: 776–791.
Vandenkoornhuyse P, Mahe S, Ineson P, Staddon P, Ostle N, Cliquet J-B et al. (2007). Active root-inhabiting microbes identified by rapid incorporation of plant-derived carbon into RNA. Proc Natl Acad Sci USA 104: 16970–16975.
Walker B . (1992). Biodiversity and ecological redundancy. Conserv Biol 6: 18–23.
Walker B . (1995). Conserving biological diversity through ecosystem resilience. Conserv Biol 9: 747–752.
Wertz S, Degrange V, Prosser JI, Poly F, Commeaux C, Freitag T et al. (2006). Maintenance of soil functioning following erosion of microbial diversity. Environ Microbiol 8: 2162–2169.
Wertz S, Degrange V, Prosser JI, Poly F, Commeaux C, Guillaumaud N et al. (2007). Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ Microbiol 9: 2211–2219.
Whiteley AS, Thomson B, Lueders T, Manefield M . (2007). RNA stable-isotope probing. Nat Protoc 2: 838–844.
Yachi S, Loreau M . (1999). Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA 96: 1463–1468.
Acknowledgements
This work was supported by funding from the INSU-CNRS EC2CO programme and by a grant from the council of ‘Région Bretagne’ to C Monard and F Binet. C Rouillon and C Briens from EHESP/LERES are gratefully acknowledged for their technical support throughout this study. We are also grateful to Dr N Nunan, Dr F Nunes, Dr A Dufresne and Dr A Quaiser for their insight and valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Monard, C., Vandenkoornhuyse, P., Le Bot, B. et al. Relationship between bacterial diversity and function under biotic control: the soil pesticide degraders as a case study. ISME J 5, 1048–1056 (2011). https://doi.org/10.1038/ismej.2010.194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.194
Keywords
This article is cited by
-
Change in microbial profile and environmental conditions in a constructed wetland system treating greywater
Environmental Science and Pollution Research (2021)
-
Defining lower limits of biodegradation: atrazine degradation regulated by mass transfer and maintenance demand in Arthrobacter aurescens TC1
The ISME Journal (2019)
-
Influx and concentration of triazine pesticides in the Amaterska cave system, Moravian Karst, Czech Republic
Journal of Soils and Sediments (2018)
-
Plant-earthworm interactions: influence of age and proportion of casts in the soil on plant growth, morphology and nitrogen uptake
Plant and Soil (2018)
-
Transcriptional profiling of wheat in response to take-all disease and mechanisms involved in earthworm’s biocontrol effect
European Journal of Plant Pathology (2016)