Abstract
Objective:
The A-allele of the fat mass and obesity-associated (FTO) gene variant rs9939609 has been associated with increased body weight, whereas no effect on weight loss during weight reduction programs has been observed. We questioned whether the AA-genotype interferes with weight stabilization after weight loss.
Design:
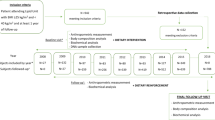
We conducted a monocentric, longitudinal study involving obese individuals. The FTO gene variant rs9939609 was genotyped in participants attending a weight reduction program that was divided into two phases: a weight reduction period with formula diet (12 weeks) and a weight maintenance phase (40 weeks). Body weight, body mass index (BMI), blood pressure and concentrations of blood glucose, total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides were determined in week 0 (T0), after 12 weeks (T1) and at the end in week 52 (T2).
Subjects:
A total of 193 obese subjects aged between 18 and 72 years (129 female, 64 male; initial body weight: 122.4±22.3 kg, initial BMI: 41.8±6.7 kg m−2) were included.
Results:
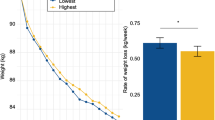
Genotyping revealed 32.1% TT-, 39.4% AT- and 28.5% AA-genotype carriers. At T0, carriers of the AA-genotype had significantly higher body weight (P=0.04) and BMI (P=0.005) than carriers of the TT-genotype. Of the 193 participants, 68 discontinued and 125 completed the program. Dropout rate was not influenced by genotype (P=0.33). Completers with AA-genotype showed significantly lower additional weight loss during the weight maintenance phase than TT-genotype carriers (P=0.02). Furthermore, among participants facing weight regain during weight maintenance (n=52), more subjects were carrying the AA-genotype (P=0.006). No influence of genotype on weight reduction under formula diet was observed (P=0.32).
Conclusion:
In this program, the AA-genotype of rs9939609 was associated with a higher initial body weight and did influence success of weight stabilization. Thus, emphasizing the maintenance phase during a weight reduction program might result in better success for AA-genotype carriers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Tsai AG, Wadden TA . Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005; 142: 56–66.
Hebebrand J, Sommerlad C, Geller F, Görg T, Hinney A . The genetics of obesity: practical implications. Int J Obes Relat Metab Disord 2001; 25: 10–17.
Farooqi IS, O’Rahilly S . New advances in the genetics of early onset obesity. Int J Obes 2005; 29: 1149–1152.
Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nature Genet 2008; 40: 768–775.
Herbert A, Gerry NP, McQueen MB, Heid IM, Pfeufer A, Illig T et al. A common genetic variant is associated with adult and childhood obesity. Science 2006; 312: 279–283.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007; 316: 889–894.
Peters T, Ausmeier K, Dildrop R, Rüther U . The mouse Fused toes (Ft) mutation is the result of a 1.6-Mb deletion including the entire Iroquois B gene cluster. Mamm Genome 2002; 13: 186–188.
Peters T, Ausmeier K, Rüther U . Cloning of Fatso (Fto), a novel gene deleted by the Fused toe (Ft) mouse mutation. Mamm Genome 1999; 10: 983–986.
Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007; 39: 724–726.
Gerken T, Girard CA, Tung YCL, Webby CJ, Saudek V, Hewitson KS et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007; 318: 1469–1472.
Mei H, Chen W, Srinivasan SR, Jiang F, Schork N, Murray S et al. FTO influences on longitudinal BMI over childhood and adulthood and modulation on relationship between birth weight and longitudinal BMI. Hum Genet 2010; 128: 589–596.
Pausova Z, Syme C, Abrahamowicz M, Xiao Y, Leonard GT, Perron M et al. A common variant of the FTO gene is associated with not only increased adiposity but also elevated blood pressure in French Canadians. Circ Cardiovasc Genet 2009; 2: 260–269.
Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genetics 2007; 3: 1200–1210.
Lappalainen T, Kolehmainen M, Schwab US, Tolppanen AM, Stanèáková A, Lindström J et al. Association of the FTO gene variant (rs9939609) with cardiovascular disease in men with abnormal glucose metabolism – the Finnish Diabetes Prevention Study. Nutr Metab Cardiovasc Dis 2011; 21: 691–698.
Lappalainen TJ, Tolppanen AM, Kolehmainen M, Schwab U, Lindström J, Tuomilehto J et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study. Obesity 2009; 17: 832–836.
Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010; 42: 1086–1092.
Berulava T, Horsthemke B . The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet 2010; 18: 1054–1056.
Speakman JR, Rance KA, Johnstone AM . Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity 2008; 16: 1961–1965.
Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CAN . An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008; 359: 2558–2566.
Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr 2009; 90: 1483–1488.
Wardle J, Carnell S, Haworth CMA, Farooqi IS, O’Rahilly S, Plomin R . Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 2008; 93: 3640–3643.
Müller TD, Hinney A, Scherag A, Nguyen TT, Schreiner F, Schäfer H et al. Fat mass and obesity associated′ gene (FTO): no significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Med Genet 2008; 9: 85.
Grau K, Hansen T, Holst C, Astrup A, Saris WH, Arner P et al. Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. Int J Obes 2009; 33: 1227–1234.
Bischoff SC, Damms-Machado A, Betz C, Herpertz S, Legenbauer T, Löw T et al. Multicenter evaluation of an interdisciplinary 52-week weight loss program for obesity with regard to body weight, comorbidities and quality of life – a prospective study. Int J Obes 2011. e-pub ahead of print 14 June 2011; doi: 10.1038/ijo.2011.107.
López-Bermejo A, Petry CJ, Díaz M, Sebastiani G, de Zegher F, Dunger DB et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 2008; 93: 1501–1505.
International HapMap Project. Internet:http://hapmap.ncbi.nlm.nih.gov/.
Acknowledgements
This work was supported by a Grant of European Foundation of the Study of Diabetes (EFSD) (GR, CW) and of ERC (AdipoDif CW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Woehning, A., Schultz, JH., Roeder, E. et al. The A-allele of the common FTO gene variant rs9939609 complicates weight maintenance in severe obese patients. Int J Obes 37, 135–139 (2013). https://doi.org/10.1038/ijo.2012.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.14
Keywords
This article is cited by
-
Examining the effect of obesity-associated gene variants on breast cancer survivors in a randomized weight loss intervention
Breast Cancer Research and Treatment (2021)
-
Can Genetics Modify the Influence of Healthy Lifestyle on Lipids in the Context of Obesity and Type 2 Diabetes?
Current Cardiovascular Risk Reports (2015)
-
Gender-specific genetic associations of polymorphisms in ACE, AKR1C2, FTO and MMP2 with weight gain over a 10-year period
Genes & Nutrition (2014)
-
Obesity: Underlying Mechanisms and the Evolving Influence of Diet
Current Nutrition Reports (2012)