Abstract
Objective:
To test the hypothesis that μ-opioid receptor signaling in the nucleus accumbens contributes to hedonic (over)eating and obesity. To investigate the effects of chronic μ-opioid antagonism in the nucleus accumbens core or shell on intake of a palatable diet, and the development of diet-induced obesity in rats.
Methods and Design:
Chronic blockade of μ-opioid receptor signaling in the nucleus accumbens core or shell was achieved by means of repeated injections (every 4–5 days) of the irreversible receptor antagonist β-funaltrexamine (BFNA) over 3–5 weeks. The diet consisted of either a choice of high-fat chow, chocolate-flavored Ensure and regular chow (each nutritionally complete) or regular chow only. Intake of each food item, body weight and body fat mass were monitored throughout the study.
Results:
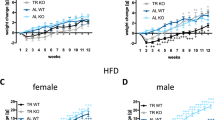
The BFNA injections aimed at either the core or shell of the nucleus accumbens resulted in significantly attenuated intake of palatable diet, body weight gain and fat accretion, compared with vehicle control injections. The injection of BFNA in the core did not significantly change these parameters in chow-fed control rats. The injection of BFNA in the core and shell differentially affected intake of the two palatable food items: in the core, BFNA significantly reduced the intake of high-fat, but not of Ensure, whereas in the shell, it significantly reduced the intake of Ensure, but not of high-fat, compared with vehicle treatment.
Conclusions:
Endogenous μ-opioid receptor signaling in the nucleus accumbens core and shell is necessary for palatable diet-induced hyperphagia and obesity to fully develop in rats. Sweet and non-sweet fatty foods may be differentially processed in subcomponents of the ventral striatum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hill JO, Wyatt HR, Reed GW, Peters JC . Obesity and the environment: where do we go from here? Science 2003; 299: 853–855.
Wyatt SB, Winters KP, Dubbert PM . Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 2006; 331: 166–174.
Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM . The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med 1998; 105: 145–150.
O’Rahilly S, Farooqi IS . Genetics of obesity. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1095–1105.
Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S . Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003; 348: 1085–1095.
Kelley AE, Berridge KC . The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 2002; 22: 3306–3311.
Lutter M, Nestler EJ . Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 2009; 139: 629–632.
Avena NM, Rada P, Hoebel BG . Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008; 32: 20–39.
Volkow ND, Wise RA . How can drug addiction help us understand obesity? Nat Neurosci 2005; 8: 555–560.
Volkow ND, Wang GJ, Fowler JS, Telang F . Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 2008; 363: 3191–3200.
Rogers PJ, Smit HJ . Food craving and food ‘addiction’: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav 2000; 66: 3–14.
Saper CB, Chou TC, Elmquist JK . The need to feed: homeostatic and hedonic control of eating. Neuron 2002; 36: 199–211.
Berthoud HR, Morrison C . The brain, appetite, and obesity. Annu Rev Psychol 2008; 59: 55–92.
Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M . Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 2002; 76: 365–377.
Kelley AE, Bless EP, Swanson CJ . Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther 1996; 278: 1499–1507.
Will MJ, Franzblau EB, Kelley AE . Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 2003; 23: 2882–2888.
Zheng H, Patterson LM, Berthoud HR . Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 2007; 27: 11075–11082.
Zhang M, Gosnell BA, Kelley AE . Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 1998; 285: 908–914.
Zheng H, Patterson LM, Berthoud HR . Orexin-signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 2007; 27: 11075–11082.
Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR . Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1436–R1444.
Groenewegen HJ, Russchen FT . Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol 1984; 223: 347–367.
Otake K, Nakamura Y . Possible pathways through which neurons of the shell of the nucleus accumbens influence the outflow of the core of the nucleus accumbens. Brain Dev 2000; 22 (Suppl 1): S17–S26.
Sano H, Yokoi M . Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci 2007; 27: 6948–6955.
Usuda I, Tanaka K, Chiba T . Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 1998; 797: 73–93.
Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE . A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem 1980; 23: 233–234.
Ward SJ, Portoghese PS, Takemori AE . Improved assays for the assessment of kappa- and delta-properties of opioid ligands. Eur J Pharmacol 1982; 85: 163–170.
Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ . Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci 2006; 23: 1605–1613.
Ward HG, Simansky KJ . Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 2006; 187: 435–446.
Arjune D, Standifer KM, Pasternak GW, Bodnar RJ . Reduction by central beta-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res 1990; 535: 101–109.
Cole JL, Leventhal L, Pasternak GW, Bowen WD, Bodnar RJ . Reductions in body weight following chronic central opioid receptor subtype antagonists during development of dietary obesity in rats. Brain Res 1995; 678: 168–176.
Cole JL, Berman N, Bodnar RJ . Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides 1997; 18: 1201–1207.
Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML . General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res 1995; 700: 205–212.
Ragnauth A, Moroz M, Bodnar RJ . Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res 2000; 876: 76–87.
Znamensky V, Echo JA, Lamonte N, Christian G, Ragnauth A, Bodnar RJ . gamma-Aminobutyric acid receptor subtype antagonists differentially alter opioid-induced feeding in the shell region of the nucleus accumbens in rats. Brain Res 2001; 906: 84–91.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates 2nd edn Academic Press: San Diego, 1986.
Nicholson C . Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res 1985; 333: 325–329.
Martin WR, Wikler A, Eades CG, Pescor FT . Tolerance to and physical dependence on morphine in rats. Psychopharmacologia 1963; 4: 247–260.
South T, Deng C, Huang XF . AM 251 and beta-funaltrexamine reduce fat intake in a fat-preferring strain of mouse. Behav Brain Res 2007; 181: 153–157.
Holtzman SG . Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther 1974; 189: 51–60.
Bodnar RJ . Endogenous opiates and behavior: 2006. Peptides 2007; 28: 2435–2513.
Statnick MA, Tinsley FC, Eastwood BJ, Suter TM, Mitch CH, Heiman ML . Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1399–R1408.
Kotz CM, Billington CJ, Levine AS . Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol 1997; 272: R1028–R1032.
Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ . An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 2003; 285: R1055–R1065.
Vaccarino FJ, Taube MR . Intra-arcuate opiate actions stimulate GRF-dependent and protein-selective feeding. Peptides 1997; 18: 197–205.
Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ . Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol 2008; 295: R388–R394.
McLean S, Hoebel BG . Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides 1983; 4: 287–292.
Mucha RF, Iversen SD . Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res 1986; 397: 214–224.
MacDonald AF, Billington CJ, Levine AS . Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol 2003; 285: R999–R1004.
Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a ‘thrifty gene’. Diabetes 2005; 54: 3510–3516.
Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR . Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur J Pharmacol 2008; 585: 14–23.
Zhang M, Kelley AE . Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997; 132: 350–360.
Levine AS, Billington CJ . Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav 2004; 82: 57–61.
Leventhal L, Cole JL, Bodnar RJ . Reductions in locomotor activity following central opioid receptor subtype antagonists in rats. Physiol Behav 1996; 60: 833–836.
Levin BE, Keesey RE . Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol 1998; 274: R412–R419.
Zhang M, Kelley AE . Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience 2000; 99: 267–277.
Pecina S, Berridge KC . Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 2005; 25: 11777–11786.
Acknowledgements
This study was supported by National Institutes of Diabetes and Digestive and Kidney Disease Grant DK071082.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lenard, N., Zheng, H. & Berthoud, HR. Chronic suppression of μ-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes 34, 1001–1010 (2010). https://doi.org/10.1038/ijo.2009.297
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.297
Keywords
This article is cited by
-
Central effects of opioidergic system on food intake in birds and mammals: a review
Veterinary Research Communications (2023)
-
Methocinnamox (MCAM) antagonizes the behavioral suppressant effects of morphine without impairing delayed matching-to-sample accuracy in rhesus monkeys
Psychopharmacology (2020)
-
Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity
Neuropsychopharmacology (2015)
-
Hormonal and neural mechanisms of food reward, eating behaviour and obesity
Nature Reviews Endocrinology (2014)
-
Inhibition of Opioid Transmission at the μ-Opioid Receptor Prevents Both Food Seeking and Binge-Like Eating
Neuropsychopharmacology (2012)