Abstract
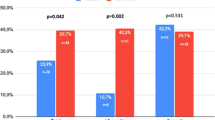
Initiation of ED treatment with a particular PDE5I may influence treatment-adherence and other outcomes. In this multicenter, open-label study, men with ED, naïve to PDE5I, were randomized to tadalafil 5 mg once-a-day (OaD; N=257), 10 mg on demand (PRN; N=252) or sildenafil-citrate (sildenafil) 50 mg PRN (N=261) for 8 weeks (dose adjustments allowed), followed by 16 weeks of pragmatic treatment (switching between PDE5I allowed). Primary outcomes (treatment-adherence) were reported previously. Here, we report effects on: Psychological and Interpersonal Relationship Scales, Self-Esteem and Relationship (SEAR) questionnaire, ED Inventory of Treatment Satisfaction (EDITS), International Index of Erectile Function (IIEF), Sexual Encounter Profile (SEP) and Global Assessment Questions (GAQ). Mixed-model for repeated measures and analysis of covariance were used to analyze changes from baseline; GAQ-responses were evaluated by logistic regression. Analyses were adjusted for treatment, country, ED-severity, baseline and baseline-by-treatment interaction. Patients randomized to tadalafil OaD or PRN reported greater improvement (least-square mean (s.e.) change) in Sexual Self-Confidence (OaD +0.90 (0.048), PRN +0.93 (0.050), vs +0.73 (0.049); P=0.006 and P=0.001) and Spontaneity (OaD +0.11 (0.035), PRN +0.13 (0.035), vs +0.02 (0.035); P=0.044 and P=0.010) compared with sildenafil. Improvements in GAQ and SEP responses, IIEF-EF, orgasmic function, sexual desire, overall satisfaction domains, SEAR and EDITS scores did not differ significantly between treatment groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA . Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998; 338: 1397–1404.
Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol 2002; 168: 1332–1336.
Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, Taylor T et al. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl 2002; 23: 763–771.
Porst H, Rajfer J, Casabé A, Feldman R, Ralph D, Vieiralves LF et al. Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med 2008; 5: 2160–2169.
Montorsi F, Aversa A, Moncada I, Perimenis P, Porst H, Barker C et al. A randomized, double-blind, placebo-controlled, parallel study to assess the efficacy and safety of once-a-day tadalafil in men with erectile dysfunction who are naïve to PDE5 inhibitors. J Sex Med 2011; 8: 2617–2624.
Tsertsvadze A, Fink HA, Yazdi F, MacDonald R, Bella AJ, Ansari MT et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med 2009; 151: 650–661.
Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M . The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin 2004; 20: 605–617.
Hatzichristou D, Haro J, Martin-Morales A, von Keitz A, Riley A, Bertsch J et al. Patterns of switching PDE5 inhibitors in the treatment of erectile dysfunction: results from the Erectile Dysfunction Observational Study. Int J Clin Pract 2007; 61: 1850–1862.
Buvat J, Büttner H, Hatzimouratidis K, Vendeira P, Moncada I, Boehmer M et al. Adherence to initial PDE5 inhibitor treatment: randomized open-label study comparing tadalafil once a day, tadalafil on demand and sildenafil on demand in patients with erectile dysfunction. J Sex Med 2013; 10: 1592–1602.
Walker DK, Ackland MJ, James GC, Muirhead GJ, Rance DJ, Wastall P et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica 1999; 29: 297–310.
Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2006; 61: 280–288.
Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R . Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology 2003; 62: 121–125.
Young JM, Feldman RA, Auerbach SM, Kaufman JM, Garcia CS, Shen W et al. Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J Androl 2005; 26: 310–318.
Gingell C, Sultana SR, Wulff MB, Gepi-Attee S . Duration of action of sildenafil citrate in men with erectile dysfunction. J Sex Med 2004; 1: 179–184.
Wrishko R, Sorsaburu S, Wong D, Strawbridge A, McGill J . Safety, efficacy, and pharmacokinetic overview of low-dose daily administration of tadalafil. J Sex Med 2009; 6: 2039–2048.
Rajfer J, Aliotta PJ, Steidle CP, Fitch WP 3rd, Zhao Y, Yu A . Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res 2007; 19: 95–103.
Althof SE . When an erection alone is not enough: biopsychosocial obstacles to lovemaking. Int J Impot Res 2002; 14 (Suppl 1): S99–S104.
Dean J, Hackett GI, Gentile V, Pirozzi-Farina F, Rosen RC, Zhao Y et al. Psychosocial outcomes and drug attributes affecting treatment choice in men receiving sildenafil citrate and tadalafil for the treatment of erectile dysfunction: results of a multicenter, randomized, open-label, crossover study. J Sex Med 2006; 3: 650–661.
Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F et al. European Association of Urology. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010; 57: 804–814.
Swindle RW, Cameron AE, Lockhart DC, Rosen RC . The psychological and interpersonal relationship scales: assessing psychological and relationship outcomes associated with erectile dysfunction and its treatment. Arch Sex Behav 2004; 33: 19–30.
Cappelleri JC, Althof SE, Siegel RL, Shpilsky A, Bell SS, Duttagupta S . Development and validation of the Self-Esteem And Relationship (SEAR) questionnaire in erectile dysfunction. Int J Impot Res 2004; 16: 30–38.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A . The international index of erectile function (IIEF): a multidimensional scale for the assessment of erectile dysfunction. Urology 1997; 49: 822–830.
Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH . Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999; 54: 346–351.
Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, McVary K et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology 1999; 53: 793–799.
Rubio-Aurioles E, Porst H, Kim ED, Montorsi F, Hackett G, Morales AM et al. A randomized open-label trial with a crossover comparison of sexual self-confidence and other treatment outcomes following tadalafil once a day vs tadalafil or sildenafil on-demand in men with erectile dysfunction. J Sex Med 2012; 9: 1418–1429.
El Khiat Y, Ghazi S, Allam A, Khawaja M, Belger M, Tamer M et al. Psychosocial impact and effectiveness of tadalafil among treatment-naïve and previously-treated men with erectile dysfunction in Saudi Arabia and other Gulf-region countries. Curr Med Res Opin 2008; 24: 1965–1973.
Acknowledgements
We thank all patients who volunteered to participate in this trial and all study investigators for their contribution to data acquisition and patient care. We thank Beatriz Sanz, Eli Lilly and Company, Spain, and Krzysztof Jasek, Eli Lilly and Company, Poland, for supporting the conduct of the study. We thank Clare Barker, Lilly UK, Global Statistical Sciences, UK, and Bruce Basson, Lilly France, Global Statistical Sciences, Suresnes, France, for their contributions to the statistical analysis plan. We thank Karin Helsberg, PhD, and Michael Riley, PhD, Trilogy Writing and Consulting GmbH, Frankfurt, Germany, for providing medical writing services on behalf of Eli Lilly and Company. Statistical analyses were programmed by PSI CRO LTD, St Petersburg, Russia. The study was funded by Eli Lilly and Company, Indianapolis, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
KH has served as speaker for Eli Lilly. JB has received honorary as scientific advisor for Eli Lilly and has been clinical trial investigator for trials sponsored by Eli Lilly, Bayer-Schering, Boehringer Ingelheim and Janssen-Cilag. IM has served as scientific advisor and speaker for Janssen-Cilag, Eli Lilly and Co., Bayer-Schering; and has received research grants from Pfizer. IM, KH, MB and PASV have been clinical trial investigator for trials sponsored by Eli Lilly. HB, CH and FGB are employees of Eli Lilly and Company; HB and FGB also own Lilly stock.
Rights and permissions
About this article
Cite this article
Hatzimouratidis, K., Buvat, J., Büttner, H. et al. Psychosocial outcomes after initial treatment of erectile dysfunction with tadalafil once daily, tadalafil on demand or sildenafil citrate on demand: results from a randomized, open-label study. Int J Impot Res 26, 223–229 (2014). https://doi.org/10.1038/ijir.2014.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2014.15
This article is cited by
-
Efficacy and safety of oral phosphodiesterase 5 inhibitors for erectile dysfunction: a network meta-analysis and multicriteria decision analysis
World Journal of Urology (2021)
-
Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: a systematic review and meta-analysis
International Urology and Nephrology (2017)
-
Psychosocial perspectives on sexual recovery after prostate cancer treatment
Nature Reviews Urology (2015)