Abstract
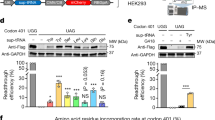
An emerging strategy for the treatment of monogenic diseases uses genetic engineering to precisely correct the mutation(s) at the genome level. Recent advancements in this technology have demonstrated therapeutic levels of gene correction using a zinc-finger nuclease (ZFN)-induced DNA double-strand break in conjunction with an exogenous DNA donor substrate. This strategy requires efficient nucleic acid delivery and among viral vectors, recombinant adeno-associated virus (rAAV) has demonstrated clinical success without pathology. However, a major limitation of rAAV is the small DNA packaging capacity and to date, the use of rAAV for ZFN gene delivery has yet to be reported. Theoretically, an ideal situation is to deliver both ZFNs and the repair substrate in a single vector to avoid inefficient gene targeting and unwanted mutagenesis, both complications of a rAAV co-transduction strategy. Therefore, a rAAV format was generated in which a single polypeptide encodes the ZFN monomers connected by a ribosome skipping 2A peptide and furin cleavage sequence. On the basis of this arrangement, a DNA repair substrate of 750 nucleotides was also included in this vector. Efficient polypeptide processing to discrete ZFNs is demonstrated, as well as the ability of this single vector format to stimulate efficient gene targeting in a human cell line and mouse model derived fibroblasts. Additionally, we increased rAAV-mediated gene correction up to sixfold using a combination of Food and Drug Administration-approved drugs, which act at the level of AAV vector transduction. Collectively, these experiments demonstrate the ability to deliver ZFNs and a repair substrate by a single AAV vector and offer insights for the optimization of rAAV-mediated gene correction using drug therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M . 20 years of gene therapy for SCID. Nat Immunol 2010; 11: 457–460.
Cappelli B, Aiuti A . Gene therapy for adenosine deaminase deficiency. Immunol Allergy Clin North Am 2010; 30: 249–260.
Qasim W, Gaspar HB, Thrasher AJ . Progress and prospects: gene therapy for inherited immunodeficiencies. Gene Therapy 2009; 16: 1285–1291.
Liu MM, Tuo J, Chan CC . Gene therapy for ocular diseases. Br J Ophthalmol 2011; 95: 604–612.
Porteus MH, Baltimore D . Chimeric nucleases stimulate gene targeting in human cells. Science 2003; 300: 763.
Sedivy JM, Dutriaux A . Gene targeting and somatic cell genetics--a rebirth or a coming of age? Trends Genet 1999; 15: 88–90.
Brenneman M, Gimble FS, Wilson JH . Stimulation of intrachromosomal homologous recombination in human cells by electroporation with site-specific endonucleases. Proc Natl Acad Sci USA 1996; 93: 3608–3612.
Choulika A, Perrin A, Dujon B, Nicolas JF . Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 1995; 15: 1968–1973.
Donoho G, Jasin M, Berg P . Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol Cell Biol 1998; 18: 4070–4078.
Rouet P, Smih F, Jasin M . Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol 1994; 14: 8096–8106.
Sargent RG, Brenneman MA, Wilson JH . Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol Cell Biol 1997; 17: 267–277.
Smih F, Rouet P, Romanienko PJ, Jasin M . Double-strand breaks at the target locus stimulate gene targeting in embryonic stem cells. Nucleic Acids Res 1995; 23: 5012–5019.
Taghian DG, Nickoloff JA . Chromosomal double-strand breaks induce gene conversion at high frequency in mammalian cells. Mol Cell Biol 1997; 17: 6386–6393.
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010; 186: 757–761.
Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 2011; 29: 143–148.
Paques F, Duchateau P . Meganucleases and DNA double-strand break-induced recombination: perspectives for gene therapy. Curr Gene Ther 2007; 7: 49–66.
Porteus MH, Carroll D . Gene targeting using zinc finger nucleases. Nat Biotechnol 2005; 23: 967–973.
Smith J, Berg JM, Chandrasegaran S . A detailed study of the substrate specificity of a chimeric restriction enzyme. Nucleic Acids Res 1999; 27: 641–681.
Carroll D, Beumer KJ, Trautman JK . High-efficiency gene targeting in Drosophila with zinc finger nucleases. Methods Mol Biol 2010; 649: 271–280.
Connelly JP, Barker JC, Pruett-Miller S, Porteus MH . Gene correction by homologous recombination with zinc finger nucleases in primary cells from a mouse model of a generic recessive genetic disease. Mol Ther 2010; 18: 1103–1110.
Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol 2010; 28: 839–847.
Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 2007; 25: 1298–1306.
Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol 2008; 26: 808–816.
Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 2005; 435: 646–651.
Michelfelder S, Trepel M . Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet 2009; 67: 29–60.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE . Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008; 16: 1073–1080.
Hendrie PC, Russell DW . Gene targeting with viral vectors. Mol Ther 2005; 12: 9–17.
Miller DG, Petek LM, Russell DW . Human gene targeting by adeno-associated virus vectors is enhanced by DNA double-strand breaks. Mol Cell Biol 2003; 23: 3550–3557.
Porteus MH, Cathomen T, Weitzman MD, Baltimore D . Efficient gene targeting mediated by adeno-associated virus and DNA double-strand breaks. Mol Cell Biol 2003; 23: 3558–3565.
Gellhaus K, Cornu TI, Heilbronn R, Cathomen T . Fate of recombinant adeno-associated viral vector genomes during DNA double-strand break-induced gene targeting in human cells. Hum Gene Ther 2010; 21: 543–553.
Dekelver RC, Choi VM, Moehle EA, Paschon DE, Hockemeyer D, Meijsing SH et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res 2010; 20: 1133–1142.
Orlando SJ, Santiago Y, Dekelver RC, Freyvert Y, Boydston EA, Moehle EA et al. Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology. Nucleic Acids Res 2010; 38: e152.
Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Therapy 2008; 15: 1411–1423.
Pruett-Miller SM, Connelly JP, Maeder ML, Joung JK, Porteus MH . Comparison of zinc finger nucleases for use in gene targeting in mammalian cells. Mol Ther 2008; 16: 707–717.
Pruett-Miller SM, Reading DW, Porter SN, Porteus MH . Attenuation of zinc finger nuclease toxicity by small-molecule regulation of protein levels. PLoS Genet 2009; 5: e1000376.
Johnson JS, Samulski RJ . Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol 2009; 83: 2632–2644.
Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther 2010; 18: 1907–1916.
Denby L, Nicklin SA, Baker AH . Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Therapy 2005; 12: 1534–1538.
Douar AM, Poulard K, Stockholm D, Danos O . Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol 2001; 75: 1824–1833.
Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF . Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000; 105: 1573–1587.
Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther 2010; 18: 135–142.
Jennings K, Miyamae T, Traister R, Marinov A, Katakura S, Sowders D et al. Proteasome inhibition enhances AAV-mediated transgene expression in human synoviocytes in vitro and in vivo. Mol Ther 2005; 11: 600–607.
Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF . Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002; 76: 2043–2053.
Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007; 317: 477.
Kay MA, Nakai H . Looking into the safety of AAV vectors. Nature 2003; 424: 251.
Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA . AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet 2003; 34: 297–302.
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 2007; 369: 2097–2105.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009; 374: 1597–1605.
Maguire AM, Simonelli F, Pierce EA, Pugh Jr EN, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol Ther 2010; 18: 1731–1735.
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010; 18: 643–650.
Sun X, Pawlyk B, Xu X, Liu X, Bulgakov OV, Adamian M et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Therapy 2010; 17: 117–131.
Kingston RE, Chen CA, Okayama H . Calcium phosphate transfection. Curr Protoc Immunol 2001, Chapter 10, Unit 10 13: 10.13.1–10.13.9.
Rao NM . Cationic lipid-mediated nucleic acid delivery: beyond being cationic. Chem Phys Lipids 2010; 163: 245–252.
Cornu TI, Cathomen T . Targeted genome modifications using integrase-deficient lentiviral vectors. Mol Ther 2007; 15: 2107–2113.
Cathomen T, Joung JK . Zinc-finger nucleases: the next generation emerges. Mol Ther 2008; 16: 1200–1207.
Hirsch ML, Green L, Porteus MH, Samulski RJ . Self-complementary AAV mediated gene targeting and enhances endonuclease delivery for double-strand break repair. Gene Therapy 2010; 19: 1175–1180.
Dong X, Tian W, Wang G, Dong Z, Sen W, Zheng G et al. Establishment of AAV reverse infection-based array. PloS One 2010; 5: e13479.
Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 2011; 475: 217–221.
Grieger JC, Choi VW, samulski RJ . Production and characterization of adeno-associated viral vectors. Nat Protoc 2006; 1: 1412–1428.
Acknowledgements
We thank Kelley Ellis for her helpful review and comments. This work was funded by the Northwest Genome Engineering Consortium (NGEC) to support MH. Work in the Porteus lab is supported by funds from the state of Texas, a career development award from the Burroughs-Wellcome fund, the Amon Carter Foundation and grant PN2EY018244 supporting a Nanomedicine Development Center through the Director's Common Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Ellis, B., Hirsch, M., Porter, S. et al. Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs. Gene Ther 20, 35–42 (2013). https://doi.org/10.1038/gt.2011.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.211
Keywords
This article is cited by
-
Prospects of viral vector-mediated delivery of sequences encoding anti-HBV designer endonucleases
Gene Therapy (2022)
-
Use of gene-editing technology to introduce targeted modifications in pigs
Journal of Animal Science and Biotechnology (2018)
-
Genome editing for the treatment of tumorigenic viral infections and virus-related carcinomas
Frontiers of Medicine (2018)
-
A history of genome editing in mammals
Mammalian Genome (2017)
-
Engineered Viruses as Genome Editing Devices
Molecular Therapy (2016)