Key Points
-
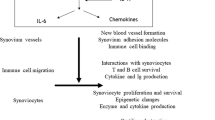
Autoreactive T cells have an important role in the pathogenesis of both rheumatoid arthritis (RA) and mouse models of the disease
-
In addition to similarities, some differences exist in the regulation of the development and plasticity of type 1 and type 17 T-helper cell and regulatory T-cell subsets in humans and mice
-
The contribution of joint-homing T cells to local inflammation in arthritis is not clear in either species
-
Unlike in mouse arthritis, most T-cell-targeting therapeutic strategies failed in RA; only a few of them (such as abatacept) succeeded
-
Anti-cytokine and anti-B-cell therapies might indirectly suppress pathogenic T cells in human and mouse arthritis
Abstract
The involvement of autoreactive T cells in the pathogenesis of rheumatoid arthritis (RA) as well as in autoimmune animal models of arthritis has been well established; however, unanswered questions, such as the role of joint-homing T cells, remain. Animal models of arthritis are superb experimental tools in demonstrating how T cells trigger joint inflammation, and thus can help to further our knowledge of disease mechanisms and potential therapies. In this Review, we discuss the similarities and differences in T-cell subsets and functions between RA and mouse arthritis models. For example, various T-cell subsets are involved in both human and mouse arthritis, but differences might exist in the cytokine regulation and plasticity of these cells. With regard to joint-homing T cells, an abundance of synovial T cells is present in humans compared with mice. On the other hand, local expansion of type 17 T-helper (TH17) cells is observed in some animal models, but not in RA. Finally, whereas T-cell depletion therapy essentially failed in RA, antibody targeting of T cells can work, at least preventatively, in most arthritis models. Clearly, additional human and animal studies are needed to fill the gap in our understanding of the specific contribution of T-cell subsets to arthritis in mice and men.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kamradt, T. & Frey, O. Arthritis: where are the T cells? Arthritis Res. Ther. 12, 122 (2010).
Angyal, A. et al. Development of proteoglycan-induced arthritis depends on T cell-supported autoantibody production, but does not involve significant influx of T cells into the joints. Arthritis Res. Ther. 12, R44 (2010).
Szekanecz, Z. & Koch, A. E. Update on synovitis. Curr. Rheumatol. Rep. 3, 53–63 (2001).
Wehrens, E. J., Prakken, B. J. & van Wijk, F. T cells out of control—impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 9, 34–42 (2013).
Wooley, P. H. Animal models of rheumatoid arthritis. Curr. Opin. Rheumatol. 3, 407–420 (1991).
Alzabin, S. & Williams, R. O. Effector T cells in rheumatoid arthritis: lessons from animal models. FEBS Lett. 585, 3649–3659 (2011).
Glant, T. T., Mikecz, K., Arzoumanian, A. & Poole, A. R. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 30, 201–212 (1987).
Glant, T. T., Finnegan, A. & Mikecz, K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit. Rev. Immunol. 23, 199–250 (2003).
Misjak, P. et al. The role of citrullination of an immunodominant proteoglycan (PG) aggrecan T cell epitope in BALB/c mice with PG-induced arthritis. Immunol. Lett. 152, 25–31 (2013).
Sakaguchi, S., Takahashi, T., Hata, T., Nomura, T. & Sakaguchi, N. SKG mice, a new genetic model of rheumatoid arthritis. Arthritis Res. Ther. 5 (Suppl. 3), 10 (2003).
Banerjee, S., Webber, C. & Poole, A. R. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles of CD4+ and CD8+ T cells. Cell. Immunol. 144, 347–357 (1992).
Goldschmidt, T. J., Andersson, M., Malmstrom, V. & Holmdahl, R. Activated type II collagen reactive T cells are not eliminated by in vivo anti-CD4 treatment. Implications for therapeutic approaches on autoimmune arthritis. Immunobiology 184, 359–371 (1992).
Kadowaki, K. M., Matsuno, H., Tsuji, H. & Tunru, I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin. Exp. Immunol. 97, 212–218 (1994).
Schubert, D., Maier, B., Morawietz, L., Krenn, V. & Kamradt, T. Immunization with glucose-6-phosphate isomerase induces T cell-dependent peripheral polyarthritis in genetically unaltered mice. J. Immunol. 172, 4503–4509 (2004).
Thomas, R., Turner, M. & Cope, A. P. High avidity autoreactive T cells with a low signalling capacity through the T-cell receptor: central to rheumatoid arthritis pathogenesis? Arthritis Res. Ther. 10, 210 (2008).
Cope, A. P. Altered signalling thresholds in T lymphocytes cause autoimmune arthritis. Arthritis Res. Ther. 6, 112–116 (2004).
Snir, O. et al. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheum. 64, 2482–2488 (2012).
Law, S. C. et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res. Ther. 14, R118 (2012).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Kurko, J. et al. Genetics of rheumatoid arthritis—a comprehensive review. Clin. Rev. Allergy Immunol. 45, 170–179 (2013).
Brand, D. D. et al. Autoantibodies to murine type II collagen in collagen-induced arthritis: a comparison of susceptible and nonsusceptible strains. J. Immunol. 157, 5178–5184 (1996).
Korganow, A. S. et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 10, 451–461 (1999).
Kouskoff, V. et al. Organ-specific disease provoked by systemic autoimmunity. Cell 87, 811–822 (1996).
Park, S. H. et al. Shift toward T helper 1 cytokines by type II collagen-reactive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 44, 561–569 (2001).
von Delwig, A., Locke, J., Robinson, J. H. & Ng, W. F. Response of TH17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 62, 143–149 (2010).
Masson-Bessiere, C. et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the α- and β-chains of fibrin. J. Immunol. 166, 4177–4184 (2001).
Cordova, K. N., Willis, V. C., Haskins, K. & Holers, V. M. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J. Immunol. 190, 1457–1465 (2013).
Waase, I., Kayser, C., Carlson, P. J., Goronzy, J. J. & Weyand, C. M. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 39, 904–913 (1996).
Schirmer, M., Vallejo, A. N., Weyand, C. M. & Goronzy, J. J. Resistance to apoptosis and elevated expression of BCL-2 in clonally expanded CD4+CD28− T cells from rheumatoid arthritis patients. J. Immunol. 161, 1018–1025 (1998).
Miossec, P. & van den Berg, W. TH1/TH2 cytokine balance in arthritis. Arthritis Rheum. 40, 2105–2115 (1997).
De Carli, M., D'Elios, M. M., Zancuoghi, G., Romagnani, S. & Del Prete, G. Human TH1 and TH2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity 18, 301–308 (1994).
Laurence, A. & O'Shea, J. J. TH17 differentiation: of mice and men. Nat. Immunol. 8, 903–905 (2007).
van den Berg, W. B. & Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 549–553 (2009).
Janson, P. C. et al. Profiling of CD4+ T cells with epigenetic immune lineage analysis. J. Immunol. 186, 92–102 (2011).
Finnegan, A., Mikecz, K., Tao, P. & Glant, T. T. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a TH1-type disease regulated by TH2 cytokines. J. Immunol. 163, 5383–5390 (1999).
Kaplan, C. et al. TH1 and TH2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 4, 54–58 (2002).
Hamel, K. M. et al. B cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J. Immunol. 187, 4900–4906 (2011).
Hanyecz, A. et al. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the TH1 phenotype. Arthritis Rheum. 50, 1665–1676 (2004).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Nistala, K. et al. TH17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl Acad. Sci. USA 107, 14751–14756 (2010).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Doodes, P. D. et al. Development of proteoglycan-induced arthritis is independent of IL-17. J. Immunol. 181, 329–337 (2008).
Stoop, J. N., Tibbitt, C. A., van Eden, W., Robinson, J. H. & Hilkens, C. M. The choice of adjuvant determines the cytokine profile of T cells in proteoglycan-induced arthritis but does not influence disease severity. Immunology 138, 68–75 (2012).
Santarlasci, V. et al. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity 36, 201–214 (2012).
Mukasa, R. et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010).
Cohen, C. J. et al. Human TH1 and TH17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J. Immunol. 187, 5615–5626 (2011).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Chakera, A. et al. The phenotype of circulating follicular-helper T cells in patients with rheumatoid arthritis defines CD200 as a potential therapeutic target. Clin. Dev. Immunol. 2012, 948218 (2012).
Weyand, C. M. & Goronzy, J. J. Ectopic germinal center formation in rheumatoid synovitis. Ann. NY Acad. Sci. 987, 140–149 (2003).
Yu, D. & Vinuesa, C. G. The elusive identity of T follicular helper cells. Trends Immunol. 31, 377–383 (2010).
Kamphorst, A. O. & Ahmed, R. Manipulating the PD-1 pathway to improve immunity. Curr. Opin. Immunol. 25, 381–388 (2013).
Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
Raptopoulou, A. P. et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 62, 1870–1880 (2010).
Wan, B. et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 177, 8844–8850 (2006).
Hamel, K. M. et al. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. Eur J. Immunol. 40, 3117–3127 (2010).
Nakayamada, S. et al. Early TH1 cell differentiation is marked by a TFH cell-like transition. Immunity 35, 919–931 (2012).
Lin, X. et al. Advances in distinguishing natural from induced FOXP3+ regulatory T cells. Int. J. Clin. Exp. Pathol. 6, 116–123 (2013).
Flores-Borja, F., Jury, E. C., Mauri, C. & Ehrenstein, M. R. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 105, 19396–19401 (2008).
Nadkarni, S., Mauri, C. & Ehrenstein, M. R. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J. Exp. Med. 204, 33–39 (2007).
Bayry, J., Siberil, S., Triebel, F., Tough, D. F. & Kaveri, S. V. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov. Today 12, 548–552 (2007).
McGovern, J. L. et al. TH17 cells are restrained by TREG cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 64, 3129–3138 (2012).
Morgan, M. E. et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 52, 2212–2221 (2005).
Roord, S. T. et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+FOXP3+ regulatory T cells. Blood 111, 5233–5241 (2008).
Nguyen, L. T., Jacobs, J., Mathis, D. & Benoist, C. Where FOXP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 56, 509–520 (2007).
Crome, S. Q., Wang, A. Y. & Levings, M. K. Translational mini-review series on TH17 cells: function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 159, 109–119 (2010).
Afzali, B., Mitchell, P., Lechler, R. I., John, S. & Lombardi, G. Translational mini-review series on TH17 cells: induction of interleukin-17 production by regulatory T cells. Clin. Exp. Immunol. 159, 120–130 (2010).
O'Connor, R. A., Taams, L. S. & Anderton, S. M. Translational mini-review series on TH17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin. Exp. Immunol. 159, 137–147 (2010).
Valencia, X. et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006).
Nie, H. et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 19, 322–328 (2013).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Ma, H. L. et al. Tumor necrosis factor α blockade exacerbates murine psoriasis-like disease by enhancing TH17 function and decreasing expansion of TREG cells. Arthritis Rheum. 62, 430–440 (2010).
Herrath, J. et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur. J. Immunol. 41, 2279–2290 (2011).
Koenen, H. J. et al. Human CD25highFOXP3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 (2008).
van Amelsfort, J. M., Jacobs, K. M., Bijlsma, J. W., Lafeber, F. P. & Taams, L. S. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 50, 2775–2785 (2004).
Gal, I. et al. Visualization and in situ analysis of leukocyte trafficking into the ankle joint in a systemic murine model of rheumatoid arthritis. Arthritis Rheum. 52, 3269–3278 (2005).
Svendsen, P. et al. Tracking of proinflammatory collagen-specific T cells in early and late collagen-induced arthritis in humanized mice. J. Immunol. 173, 7037–7045 (2004).
Szekanecz, Z., Vegvari, A., Szabo, Z. & Koch, A. E. Chemokines and chemokine receptors in arthritis. Front. Biosci. (Schol. Ed.) 2, 153–167 (2010).
Szekanecz, Z., Koch, A. E. & Tak, P. P. Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. Neth. J. Med. 69, 356–366 (2011).
Hirota, K. et al. Preferential recruitment of CCR6-expressing TH17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Loetscher, P. et al. CCR5 is characteristic of TH1 lymphocytes. Nature 391, 344–345 (1998).
Qin, S. et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 101, 746–754 (1998).
Aggarwal, A., Agarwal, S. & Misra, R. Chemokine and chemokine receptor analysis reveals elevated interferon-inducible protein-10 (IP)-10/CXCL10 levels and increased number of CCR5+ and CXCR3+ CD4 T cells in synovial fluid of patients with enthesitis-related arthritis (ERA). Clin. Exp. Immunol. 148, 515–519 (2007).
Matsui, T. et al. Selective recruitment of CCR6-expressing cells by increased production of MIP-3α in rheumatoid arthritis. Clin. Exp. Immunol. 125, 155–161 (2001).
Aerts, N. E. et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology (Oxford) 49, 2264–2272 (2010).
Kobezda, T., Ghassemi-Nejad, S., Glant, T. T. & Mikecz, K. In vivo two-photon imaging of T cell motility in joint-draining lymph nodes in a mouse model of rheumatoid arthritis. Cell. Immunol. 278, 158–165 (2012).
Szekanecz, Z. et al. Anti-citrullinated protein antibodies in rheumatoid arthritis: as good as it gets? Clin. Rev. Allergy Immunol. 34, 26–31 (2008).
Nell-Duxneuner, V. et al. Autoantibody profiling in patients with very early rheumatoid arthritis—a follow-up study. Ann. Rheum. Dis. 69, 169–174 (2010).
Klareskog, L., Widhe, M., Hermansson, M. & Ronnelid, J. Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr. Opin. Rheumatol. 20, 300–305 (2008).
Lakos, G. et al. Anti-cyclic citrullinated peptide antibody isotypes in rheumatoid arthritis: association with disease duration, rheumatoid factor production and the presence of shared epitope. Clin. Exp. Rheumatol. 26, 253–260 (2008).
Baeten, D. et al. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies. Arthritis Rheum. 44, 2255–2262 (2001).
Chang, X. et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 44, 40–50 (2005).
Caspi, D. et al. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum. 55, 53–56 (2006).
Sokolove, J. et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 7, e35296 (2012).
Fisher, B. A. et al. Heterogeneity of anticitrullinated peptide antibodies and response to anti-tumor necrosis factor agents in rheumatoid arthritis. J. Rheumatol. 39, 929–932 (2012).
Lundberg, K. et al. Antibodies to citrullinated α-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 58, 3009–3019 (2008).
Cantaert, T. et al. Alterations of the synovial T cell repertoire in anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 60, 1944–1956 (2009).
Kidd, B. A. et al. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res. Ther. 10, R119 (2008).
Glant, T. T. et al. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum. 63, 1312–1321 (2011).
Raza, K., Mullazehi, M., Salmon, M., Buckley, C. D. & Ronnelid, J. Anti-collagen type II antibodies in patients with very early synovitis. Ann. Rheum. Dis. 67, 1354–1355 (2008).
Huang, H., Benoist, C. & Mathis, D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc. Natl Acad. Sci. USA 107, 4658–4663 (2010).
Mikecz, K., Glant, T. T., Buzas, E. & Poole, A. R. Proteoglycan-induced polyarthritis and spondylitis adoptively transferred to naive (nonimmunized) BALB/c mice. Arthritis Rheum. 33, 866–876 (1990).
Keystone, E. Treatments no longer in development for rheumatoid arthritis. Ann. Rheum. Dis. 61 (Suppl. 2), ii43–ii45 (2002).
Genovese, M. C. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N. Engl. J. Med. 353, 1114–1123 (2005).
Liossis, S. N. & Sfikakis, P. P. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin. Immunol. 127, 280–285 (2008).
Feuchtenberger, M. et al. Frequency of regulatory T cells is not affected by transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Open Rheumatol. J. 2, 81–88 (2008).
Wilk, E. et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 60, 3563–3571 (2009).
Vergunst, C. E. et al. MLN3897 plus methotrexate in patients with rheumatoid arthritis: safety, efficacy, pharmacokinetics, and pharmacodynamics of an oral CCR1 antagonist in a phase IIa, double-blind, placebo-controlled, randomized, proof-of-concept study. Arthritis Rheum. 60, 3572–3581 (2009).
Egelston, C. et al. Suppression of dendritic cell maturation and t cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 64, 3179–3188 (2012).
Marijnissen, R. J. et al. Increased expression of interleukin-22 by synovial TH17 cells during late stages of murine experimental arthritis is controlled by interleukin-1 and enhances bone degradation. Arthritis Rheum. 63, 2939–2948 (2011).
Niu, X. et al. IL-21 regulates TH17 cells in rheumatoid arthritis. Hum. Immunol. 71, 334–341 (2010).
Keystone, E. C. Abandoned therapies and unpublished trials in rheumatoid arthritis. Curr. Opin. Rheumatol. 15, 253–258 (2003).
de Vries, R. Genetics of rheumatoid arthritis: time for a change! Curr. Opin. Rheumatol. 23, 227–232 (2011).
Besenyei, T. et al. Non-MHC risk alleles in rheumatoid arthritis and in the syntenic chromosome regions of corresponding animal models. Clin. Dev. Immunol. 2012, 284751 (2012).
Yang, L. et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454, 350–352 (2008).
Maddur, M. S., Miossec, P., Kaveri, S. V. & Bayry, J. TH17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 181, 8–18 (2012).
Acknowledgements
Z. Szekanecz is supported by the Medical Research Council of Hungary research grant ETT 315/2009 and by the European Union, European Social Fund and Hungary co-financed projects TÁMOP 4.2.1/B-09/1/KONV-2010-0007 and TÁMOP-4.2.2.A-11/1/KONV-2012-0031 National Excellence Convergence Program. T. T. Glant is supported by NIH grant R01 AR059356. K. Mikecz is supported by NIH grants R01 AR064206 and R21 AR062332.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to researching data for the article and writing the manuscript. In addition, K. Mikecz, T. T. Glant and Z. Szekanecz contributed to discussions of the content of the article, and T. Kobezda, K. Mikecz, T. T. Glant and Z. Szekanecz reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Kobezda, T., Ghassemi-Nejad, S., Mikecz, K. et al. Of mice and men: how animal models advance our understanding of T-cell function in RA. Nat Rev Rheumatol 10, 160–170 (2014). https://doi.org/10.1038/nrrheum.2013.205
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.205
This article is cited by
-
IgD-Fc-Ig fusion protein, a new biological agent, inhibits T cell function in CIA rats by inhibiting IgD-IgDR-Lck-NF-κB signaling pathways
Acta Pharmacologica Sinica (2020)
-
Automated Quantification of Early Bone Alterations and Pathological Bone Turnover in Experimental Arthritis by in vivo PET/CT Imaging
Scientific Reports (2017)
-
Antigen-specific immunotherapies in rheumatic diseases
Nature Reviews Rheumatology (2017)
-
Synovial tissue research: a state-of-the-art review
Nature Reviews Rheumatology (2017)
-
Acceleration of tumor growth due to dysfunction in M1 macrophages and enhanced angiogenesis in an animal model of autoimmune disease
Laboratory Investigation (2016)