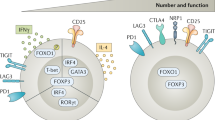
Key Points
-
T-cell co-stimulatory signals, expressed either constitutively or upon activation, critically affect the magnitude and character of autoreactive or alloreactive T-cell responses
-
Targeting T-cell co-stimulation pathways to reduce pathological T-cell responses has met with therapeutic success in many instances, but challenges remain
-
Efficacy of co-stimulatory blockade with abatacept or belatacept could be further optimized to improve inhibition of alloreactive and autoreactive T-cell responses by leaving co-inhibitory signals intact
-
Clinical application of CD154 pathway blockade has, thus far, been limited, but novel reagents in development might allow for therapeutic manipulation of this pathway to achieve immunological tolerance
-
Several other T-cell co-stimulatory pathways also hold promise as therapeutic targets for the treatment of autoimmunity and transplant rejection
-
Understanding the interplay between individual co-stimulatory and co-inhibitory pathways will lead to rational and targeted therapeutic interventions to manipulate T-cell responses and improve clinical outcomes
Abstract
The myriad of co-stimulatory signals expressed, or induced, upon T-cell activation suggests that these signalling pathways shape the character and magnitude of the resulting autoreactive or alloreactive T-cell responses during autoimmunity or transplantation, respectively. Reducing pathological T-cell responses by targeting T-cell co-stimulatory pathways has met with therapeutic success in many instances, but challenges remain. In this Review, we discuss the T-cell co-stimulatory molecules that are known to have critical roles during T-cell activation, expansion, and differentiation. We also outline the functional importance of T-cell co-stimulatory molecules in transplantation, tolerance and autoimmunity, and we describe how therapeutic blockade of these pathways might be harnessed to manipulate the immune response to prevent or attenuate pathological immune responses. Ultimately, understanding the interplay between individual co-stimulatory and co-inhibitory pathways engaged during T-cell activation and differentiation will lead to rational and targeted therapeutic interventions to manipulate T-cell responses and improve clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Salomon, B. & Bluestone, J. A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252 (2001).
Bour-Jordan, H. et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol. Rev. 241, 180–205 (2011).
Linsley, P. S. et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257, 792–795 (1992).
Lenschow, D. et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science 257, 789–792 (1992).
Finck, B. K., Linsley, P. S. & Wofsy, D. Treatment of murine lupus with CTLA4Ig. Science 265, 1225–1227 (1994).
Larsen, C. P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Wells, A. D. et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat. Med. 5, 1303–1307 (1999).
Trambley, J. et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 104, 1715–1722 (1999).
Ford, M. L. et al. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J. Immunol. 180, 7203–7211 (2008).
Adams, A. B. et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Invest. 111, 1887–1895 (2003).
Floyd, T. L. et al. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J. Immunol. 186, 2033–2041 (2011).
Bingaman, A. W. & Farber, D. L. Memory T cells in transplantation: generation, function, and potential role in rejection. Am. J. Transplant. 4, 846–852 (2004).
Yamada, Y. et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am. J. Transplant. 12, 330–340 (2012).
Ndejembi, M. P. et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J. Immunol. 177, 7698–7706 (2006).
Yuan, X. et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J. Exp. Med. 205, 3133–3144 (2008).
Ford, M. L. et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J. Exp. Med. 204, 299–309 (2007).
Genovese, M. C. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 353, 1114–1123 (2005).
Kremer, J. M. et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 349, 1907–1915 (2003).
Orban, T. et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378, 412–419 (2011).
Parulekar, A. D. et al. A randomized controlled trial to evaluate inhibition of T-cell costimulation in allergen-induced airway inflammation. Am. J. Respir. Crit. Care Med. 187, 494–501 (2013).
Linsley, P. S. & Nadler, S. G. The clinical utility of inhibiting CD28-mediated costimulation. Immunol. Rev. 229, 307–321 (2009).
Merrill, J. T. et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 3077–3087 (2010).
Sandborn, W. J. et al. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology 143, 62–69 (2012).
Larsen, C. P. et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transplant. 5, 443–453 (2005).
Vincenti, F. et al. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 353, 770–781 (2005).
Vincenti, F. et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT Study). Am. J. Transplant. 10, 535–546 (2010).
Nadazdin, O. et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci. Transl. Med. 3, 86ra51 (2011).
Burrell, B. E., Csencsits, K., Lu, G., Grabauskiene, S. & Bishop, D. K. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J. Immunol. 181, 3906–3914 (2008).
Vincenti, F. et al. Five-year safety and efficacy of belatacept in renal transplantation. J. Am. Soc. Nephrol. 21, 1587–1596 (2010).
Mellor, A. L. et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2, 3 dioxygenase. Int. Immunol. 16, 1391–1401 (2004).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774 (2004).
Munn, D. H., Sharma, M. D. & Mellor, A. L. Ligation of B7–1/B7–2 by human CD4+ T cells triggers indoleamine 2, 3-dioxygenase activity in dendritic cells. J. Immunol. 172, 4100–4110 (2004).
Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H. & Freeman, G. J. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Waibler, Z. et al. Toward experimental assessment of receptor occupancy: TGN1412 revisited. J. Allergy Clin. Immunol. 122, 890–892 (2008).
Waibler, Z. et al. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS ONE 3, e1708 (2008).
Zhang, T. et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am. J. Transplant. 11, 1599–1609 (2011).
Poirier, N. et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci. Transl. Med. 2, 17ra10 (2010).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266 (1999).
Dong, C. et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001).
Watanabe, M. et al. AP-1 is involved in ICOS gene expression downstream of TCR/CD28 and cytokine receptor signaling. Eur. J. Immunol. 42, 1850–1862 (2012).
Yu, D. et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007).
Yao, S. et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 34, 729–740 (2010).
Ansari, M. J. et al. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin. Immunol. 126, 140–147 (2008).
Sporici, R. A. et al. ICOS ligand costimulation is required for T-cell encephalitogenicity. Clin. Immunol. 100, 277–288 (2001).
Sporici, R. A. & Perrin, P. J. Costimulation of memory T-cells by ICOS: a potential therapeutic target for autoimmunity? Clin. Immunol. 100, 263–269 (2001).
Ozkaynak, E. et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat. Immunol. 2, 591–596 (2001).
Nanji, S. A. et al. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes 55, 27–33 (2006).
Schenk, A. D., Gorbacheva, V., Rabant, M., Fairchild, R. L. & Valujskikh, A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am. J. Transplant. 9, 64–73 (2009).
Hu, Y. L., Metz, D. P., Chung, J., Siu, G. & Zhang, M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J. Immunol. 182, 1421–1428 (2009).
Nurieva, R. I., Treuting, P., Duong, J., Flavell, R. A. & Dong, C. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Invest. 111, 701–706 (2003).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Paulos, C. M. et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci. Transl. Med. 2, 55ra78 (2010).
Garaude, J. & Blander, J. M. ICOStomizing immunotherapies with T(H)17. Sci. Transl. Med. 2, 55ps52 (2010).
Kirk, A. D. et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 5, 686–693 (1999).
Larsen, C. P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Ochando, J. C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7, 652–662 (2006).
Ferrer, I. R. et al. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc. Natl Acad. Sci. USA 108, 20701–20706 (2011).
Kendal, A. R. et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med. 208, 2043–2053 (2011).
Kawai, T., Andrews, D., Colvin, R. B., Sachs, D. H. & Cosimi, A. B. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 6, 114 (2000).
Monk, N. J. et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat. Med. 9, 1275–1280 (2003).
Gilson, C. R. et al. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J. Immunol. 183, 1625–1635 (2009).
Haanstra, K. G. et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation 75, 637–643 (2003).
Daley, S. R., Cobbold, S. P. & Waldmann, H. Fc-disabled anti-mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am. J. Transplant. 8, 2265–2271 (2008).
Pinelli, D. F. et al. An anti-CD154 domain antibody prolongs graft survival and induces FoxP3+ iTreg in the absence and presence of CTLA-4 Ig. Am. J. Transplant. http://dx.doi.org/10.1111/ajt.12417.
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Nimmerjahn, F., Bruhns, P., Horiuchi, K. & Ravetch, J. V. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23, 41–51 (2005).
Baudino, L. et al. Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J. Immunol. 181, 6664–6669 (2008).
Bennett, S. R. M. et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Ridge, J. P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483 (1998).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Johnson, S. et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 30, 218–227 (2009).
Bourgeois, C., Rocha, B. & Tanchot, C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297, 2060–2063 (2002).
Munroe, M. E. Functional roles for T cell CD40 in infection and autoimmune disease: the role of CD40 in lymphocyte homeostasis. Semin. Immunol. 21, 283–288 (2009).
Bhadra, R., Gigley, J. P. & Khan, I. A. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J. Immunol. 187, 4421–4425 (2011).
Liu, D., Ferrer, I. R., Konomos, M. & Ford, M. L. Inhibition of CD8+ T cell–derived CD40 signals is necessary but not sufficient for Foxp3+ induced regulatory T cell generation in vivo. J. Immunol. http://dx.doi.org/10.4049/jimmunol.1300267.
Pearson, T. C. et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 74, 933–940 (2002).
Adams, A. B. et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J. Immunol. 174, 542–550 (2005).
Thompson, P. et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am. J. Transplant. 11, 947–957 (2011).
Badell, I. R. et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. Am. J. Transplant. 12, 1918–1923 (2012).
Page, A. et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am. J. Transplant. 12, 115–125 (2012).
Lowe, M. et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am. J. Transplant. 12, 2079–2087 (2012).
Badell, I. R. et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am. J. Transplant. 12, 126–135 (2012).
Oura, T. et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am. J. Transplant. 12, 1740–1754 (2012).
Aoyagi, T. et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am. J. Transplant. 9, 1732–1741 (2009).
Imai, A. et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation 84, 1020–1028 (2007).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9, 271–285 (2009).
Vu, M. D. et al. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J. Immunol. 176, 1394–1401 (2006).
Vu, M. D. et al. OX40 costimulation turns off Foxp3+ Tregs. Blood 110, 2501–2510 (2007).
Jember, A. G., Zuberi, R., Liu, F. T. & Croft, M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J. Exp. Med. 193, 387–392 (2001).
Xiao, X. et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 13, 981–990 (2012).
Nicolls, M. R. & Gill, R. G. LFA-1 (CD11a) as a therapeutic target. Am. J. Transplant. 6, 27–36 (2006).
Springer, T. A. & Dustin, M. L. Integrin inside-out signaling and the immunological synapse. Curr. Opin. Cell Biol. 24, 107–115 (2012).
Setoguchi, K. et al. LFA-1 Antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am. J. Transplant. 11, 923–935 (2011).
Kitchens, W. H. et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am. J. Transplant. 12, 69–80 (2012).
Thompson, P. et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am. J. Transplant. 12, 1765–1775 (2012).
Poston, R. S. et al. Effects of humanized monoclonal antibody to rhesus CD11a in rhesus monkey cardiac allograft recipients. Transplantation 69, 2005–2013 (2000).
Badell, I. R. et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J. Clin. Invest. 120, 4520–4531 (2010).
Reisman, N. M. et al. LFA-1 blockade induces effector and regulatory T-cell enrichment in lymph nodes and synergizes with CTLA-4Ig to inhibit effector function. Blood 118, 5851–5861 (2011).
Singh, K. et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am. J. Transplant. 12, 1441–1457 (2012).
Lebwohl, M. et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N. Engl. J. Med. 349, 2004–2013 (2003).
Leonardi, C. L. et al. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J. Am. Acad. Dermatol. 52, 425–433 (2005).
Vincenti, F. et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am. J. Transplant. 7, 1770–1777 (2007).
Posselt, A. M. et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am. J. Transplant. 10, 1870–1880 (2010).
Turgeon, N. A. et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am. J. Transplant. 10, 2082–2091 (2010).
Carson, K. R. et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 10, 816–824 (2009).
Tan, C. S. & Koralnik, I. J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9, 425–437 (2010).
Sanders, M. E. et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J. Immunol. 140, 1401–1407 (1988).
Weaver, T. A. et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat. Med. 15, 746–749 (2009).
Moingeon, P. et al. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature 339, 312–314 (1989).
van der Merwe, P. A. A subtle role for CD2 in T cell antigen recognition. J. Exp. Med. 190, 1371–1374 (1999).
Lo, D. J. et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am. J. Transplant. 11, 22–33 (2011).
Ellis, C. N., Krueger, G. G. & Alefacept Clinical Study Group. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N. Engl. J. Med. 345, 248–255 (2001).
Brimhall, A. K., King, L. N., Licciardone, J. C., Jacobe, H. & Menter, A. Safety and efficacy of alefacept, efalizumab, etanercept and infliximab in treating moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Br. J. Dermatol. 159, 274–285 (2008).
Lowe, M. C. et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. Am. J. Transplant. 13, 312–319 (2013).
Lo, D. J. et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. Am. J. Transplant. 13, 320–328 (2013).
Rigby, M. R., Trexler, A. M., Pearson, T. C. & Larsen, C. P. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector t-cell inhibition and regulatory T-cell expansion. Diabetes 57, 2672–2683 (2008).
Kirk, A. D. et al. CTLA4Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl Acad. Sci. USA 94, 8789–8794 (1997).
Badell, I. R. et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am. J. Transplant. 12, 126–135 (2012).
Demirci, G. et al. Critical role of OX40 in CD28 and CD154-independent rejection. J. Immunol. 172, 1691–1698 (2004).
Murakawa, T. et al. Simultaneous LFA-1 and CD40 ligand antagonism prevents airway remodeling in orthotopic airway transplantation: implications for the role of respiratory epithelium as a modulator of fibrosis. J. Immunol. 174, 3869–3879 (2005).
Arefanian, H. et al. Combination of anti-CD4 with anti-LFA-1 and anti-CD154 monoclonal antibodies promotes long-term survival and function of neonatal porcine islet xenografts in spontaneously diabetic NOD mice. Cell Transplant. 16, 787–798 (2007).
Kitchens, W. H., Haridas, D., Wagener, M. E., Song, M. & Ford, M. L. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses. Transplantation 93, 997–1005 (2012).
Gibbons, C. & Sykes, M. Manipulating the immune system for anti-tumor responses and transplant tolerance via mixed hematopoietic chimerism. Immunol. Rev. 223, 334–360 (2008).
Wekerle, T. et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat. Med. 6, 464–469 (2000).
Sykes, M. Mixed chimerism and transplant tolerance. Immunity 14, 417–424 (2001).
Kean, L. S. et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am. J. Transplant. 7, 320–335 (2007).
Parker, D. C. et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl Acad. Sci. USA 92, 9560–9564 (1995).
Markees, T. G. et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J. Clin. Invest. 101, 2446–2455 (1998).
Taylor, P. A., Friedman, T. M., Korngold, R., Noelle, R. J. & Blazar, B. R. Tolerance induction of alloreactive T cells via ex vivo blockade of the CD40:CD40L costimulatory pathway results in the generation of a potent immune regulatory cell. Blood 99, 4601–4609 (2002).
Wang, T. et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J. Immunol. 180, 5991–5999 (2008).
Wang, T. et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transplant. 10, 1524–1533 (2010).
Taylor, P. A., Lees, C. J., Waldmann, H., Noelle, R. J. & Blazar, B. R. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood 98, 467–474 (2001).
Taylor, P. A., Noelle, R. J. & Blazar, B. R. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193, 1311–1318 (2001).
Kendal, A. R. et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med. 208, 2043–2053 (2011).
Zhai, Y., Meng, L., Gao, F., Busuttil, R. W. & Kupiec-Weglinski, J. W. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J. Immunol. 169, 4667–4673 (2002).
Acknowledgements
M. L. Ford is supported by R01 AI073707 and R01 AI104699. A. B. Adams and T. C. Pearson are supported by U19 AI1051731. The authors acknowledge Scott M. Krummey, Emory University, for help with conceptualization and design of figures.
Author information
Authors and Affiliations
Contributions
M. L. Ford researched the data for the article. T. C. Pearson and M. L. Ford provided substantial contribution to discussions of the content. T. C. Pearson and M. L. Ford contributed to writing the article. T. C. Pearson, M. L. Ford and A. B. Adams contributed substantially to reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
Mandy L. Ford and Andrew B. Adams have received research grants from Bristol-Myers Squibb. Thomas C. Pearson has received research grants from Bristol-Myers Squibb and Astellas.
Rights and permissions
About this article
Cite this article
Ford, M., Adams, A. & Pearson, T. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol 10, 14–24 (2014). https://doi.org/10.1038/nrneph.2013.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.183
This article is cited by
-
T follicular helper cells and T follicular regulatory cells in rheumatic diseases
Nature Reviews Rheumatology (2019)
-
Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging
Nature Biomedical Engineering (2019)
-
Belatacept and CD28 Costimulation Blockade: Preventing and Reducing Alloantibodies over the Long Term
Current Transplantation Reports (2019)
-
March1-dependent modulation of donor MHC II on CD103+ dendritic cells mitigates alloimmunity
Nature Communications (2018)
-
Inappropriate costimulation and aberrant DNA methylation as therapeutic targets in angioimmunoblastic T-cell lymphoma
Biomarker Research (2017)