Abstract
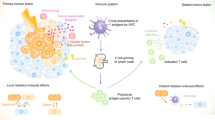
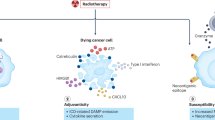
More than 60 years ago, the effect whereby radiotherapy at one site may lead to regression of metastatic cancer at distant sites that are not irradiated was described and called the abscopal effect (from 'ab scopus', that is, away from the target). The abscopal effect has been connected to mechanisms involving the immune system. However, the effect is rare because at the time of treatment, established immune-tolerance mechanisms may hamper the development of sufficiently robust abscopal responses. Today, the growing consensus is that combining radiotherapy with immunotherapy provides an opportunity to boost abscopal response rates, extending the use of radiotherapy to treatment of both local and metastatic disease. In this Opinion article, we review evidence for this growing consensus and highlight emerging limitations to boosting the abscopal effect using immunotherapy. This is followed by a perspective on current and potential cross-disciplinary approaches, including the use of smart materials to address these limitations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Atun, R. et al. Expanding global access to radiotherapy. Lancet Oncol. 16, 1153–1186 (2015).
Ngwa, W. et al. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine 9, 1063–1082 (2014).
Chang, J. Y. et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. 65, 1087–1096 (2006).
Baumann, M. et al. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 16, 234–249 (2016).
Mole, R. H. Whole body irradiation — radiobiology or medicine? Br. J. Radiol. 26, 234–241 (1953).
Andrews, J. R. The Radiobiology of Human Cancer Radiotherapy. (Univ. Park Press, 1978).
Hu, Z. I., McArthur, H. L. & Ho, A. Y. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr. Breast Cancer Rep. 9, 45–51 (2017).
Siva, S., MacManus, M. P. & Martin, R. F. & Martin, O. A. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 356, 82–90 (2013).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Wersall, P. J. et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 45, 493–497 (2006).
Ohba, K. et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 43, 575–577 (1998).
Golden, E. B. et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 16, 795–803 (2015).
Abuodeh, Y., Venkat, P. & Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 40, 25–37 (2016).
Kingsley, D. P. An interesting case of possible abscopal effect in malignant melanoma. Br. J. Radiol 48, 863–866 (1975).
Ehlers, G. & Fridman, M. Abscopal effect of radiation in papillary adenocarcinoma. Br. J. Radiol. 46, 220–222 (1973).
Stone, H. B., Peters, L. J. & Milas, L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J. Natl Cancer Inst. 63, 1229–1235 (1979).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004).
Chakravarty, P. K. et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 59, 6028–6032 (1999).
Camphausen, K. et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 63, 1990–1993 (2003).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Akutsu, Y. et al. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int. J. Oncol. 31, 509–515 (2007).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Nikitina, E. Y. & Gabrilovich, D. I. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int. J. Cancer 94, 825–833 (2001).
Kim, K. W. et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int. J. Cancer 109, 685–690 (2004).
Twyman- Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Kang, J., Demaria, S. & Formenti, S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer 4, 51 (2016).
Grass, G. D., Krishna, N. & Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 40, 10–24 (2016).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Vatner, R. E., Cooper, B. T., Vanpouille-Box, C., Demaria, S. & Formenti, S. C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 4, 325 (2014).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Habets, T. H. et al. Fractionated radiotherapy with 3 × 8 Gy induces systemic anti-tumour responses and abscopal tumour inhibition without modulating the humoral anti-tumour response. PLoS ONE 11, e0159515 (2016).
Hao, Y. et al. Enhancing radiotherapy for lung cancer using immunoadjuvants delivered in situ from new design radiotherapy biomaterials: a preclinical study. Phys. Med. Biol. 61, N697–N707 (2016).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Rodriguez-Ruiz, M. E. et al. Brachytherapy attains abscopal effects when combined with immunostimulatory monoclonal antibodies. Brachytherapy 16, 1246–1251 (2017).
Young, K. H. et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 11, e0157164 (2016).
Habets, T. H. P. M. et al. Fractionated radiotherapy with 3 × 8 Gy induces systemic anti-tumour responses and abscopal tumour inhibition without modulating the humoral anti-tumour response. PLoS ONE 11, e0159515 (2016).
Yasuda, K., Nirei, T., Tsuno, N. H., Nagawa, H. & Kitayama, J. Intratumoral injection of interleukin-2 augments the local and abscopal effects of radiotherapy in murine rectal cancer. Cancer Sci. 102, 1257–1263 (2011).
Teitz-Tennenbaum, S. et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 63, 8466–8475 (2003).
Kanegasaki, S., Matsushima, K., Shiraishi, K., Nakagawa, K. & Tsuchiya, T. Macrophage inflammatory protein derivative ECI301 enhances the alarmin-associated abscopal benefits of tumor radiotherapy. Cancer Res. 74, 5070–5078 (2014).
Marconi, R. et al. A meta-analysis of the abscopal effect in preclinical models: is the biologically effective dose a relevant physical trigger? PLoS ONE 12, e0171559 (2017).
Shiraishi, K. et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin. Cancer Res. 14, 1159–1166 (2008).
Filatenkov, A. et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 21, 3727–3739 (2015).
Bernstein, M. B., Krishnan, S., Hodge, J. W. & Chang, J. Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 13, 516–524 (2016).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Wilson, R. et al. MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nat. Commun. 7, 13597 (2016).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Dranoff, G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nat. Rev. Immunol. 12, 61–66 (2011).
McNamee, E. N., Korns Johnson, D., Homann, D. & Clambey, E. T. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 55, 58–70 (2013).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Tregcells. Nature 475, 226–230 (2011).
Doedens, A. L. et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 70, 7465–7475 (2010).
Tang, C. et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol. Res. 2, 831–838 (2014).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Picozzi, V. J., Kozarek, R. A. & Traverso, L. W. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 185, 476–480 (2003).
Picozzi, V. J. et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann. Oncol. 22, 348–354 (2011).
Bang, A. et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 98, 344–351 (2017).
Yasmin-Karim, S., Moreau, M. & Ngwa, W. in AAPM 59th Annual Meeting & Exhibition MO-DE-605-6 (Denver, CO, 2017).
Pierce, R. H., Campbell, J. S., Pai, S. I., Brody, J. D. & Kohrt, H. E. K. In-situ tumor vaccination: bringing the fight to the tumor. Hum. Vaccin. Immunother. 11, 1901–1909 (2015).
Kohrt, H. E. et al. Dose-escalated, intratumoral TLR9 agonist and low-dose radiation induce abscopal effects in follicular lymphoma. Blood 124, 3092 (2014).
Brody, J. D. et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 28, 4324–4332 (2010).
Hams, E. et al. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock 36, 295–302 (2011).
Tinganelli, W. et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci. Rep. 5, 17016 (2015).
Blankenstein, T., Coulie, P. G., Gilboa, E. & Jaffee, E. M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 12, 307–313 (2012).
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A. & Formenti, S. C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 1, 365–372 (2013).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2016).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Reply: Immunosuppressive cell death in cancer. Nat. Rev. Immunol. 17, 402–402 (2017).
Zitvogel, L. & Kroemer, G. Subversion of anticancer immunosurveillance by radiotherapy. Nat. Immunol. 16, 1005–1007 (2015).
Ma, Y. et al. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 30, 71–82 (2011).
Gorin, J.-B. et al. Antitumor immunity induced after α irradiation. Neoplasia 16, 319–328 (2014).
Zheng, W. et al. Combination of radiotherapy checkpoint blockade resistance and vaccination overcomes checkpoint blockade resistance. Oncotarget 7, 43039–43051 (2016).
Le, D. T. et al. A live-attenuated listeria vaccine (ANZ-100) and a live-attenuated listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin. Cancer Res. 18, 858–868 (2012).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Garnett-Benson, C., Hodge, J. W. & Gameiro, S. R. Combination regimens of radiation therapy and therapeutic cancer vaccines: mechanisms and opportunities. Semin. Radiat. Oncol. 25, 46–53 (2015).
Ngwa, W. et al. Smart radiation therapy biomaterials. Int. J. Radiat. Oncol. 97, 624–637 (2017).
Toy, R. & Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl Med. 1, 47–62 (2016).
Thomas, S. N., Vokali, E., Lund, A. W., Hubbell, J. A. & Swartz, M. A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 (2014).
Li, S. Y. et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 231, 17–28 (2016).
Kim, M. S. et al. Gold nanoparticles enhance anti-tumor effect of radiotherapy to hypoxic tumor. Radiat. Oncol. J. 34, 230–238 (2016).
Sinha, N. et al. Brachytherapy application with in situ dose painting administered by gold nanoparticle eluters. Int. J. Radiat. Oncol. Biol. Phys. 91, 385–392 (2015).
Detappe, A. et al. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 6, 4 (2015).
Luchette, M., Korideck, H., Makrigiorgos, M., Tillement, O. & Berbeco, R. Radiation dose enhancement of gadolinium-based AGuIX nanoparticles on HeLa cells. Nanomedicine 10, 1751–1755 (2014).
Low, D. A. in Advances in Radiation Oncology (eds Wong, J. Y. C., Schultheiss, T. E. & Radany, E. H.) 41–67 (Springer, 2017).
Lux, F. et al. Gadolinium-based nanoparticles for theranostic MRI-radiosensitization. Nanomedicine 10, 1801–1815 (2015).
Meir, R. et al. Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano 9, 6363–6372 (2015).
Kalbasi, A., June, C. H., Haas, N. & Vapiwala, N. Radiation and immunotherapy: a synergistic combination. J. Clin. Invest. 123, 2756–2763 (2013).
DeMuth, P. C. et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat. Biotechnol. 31, 1082–1085 (2013).
Park, J. & Babensee, J. E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 8, 3606–3617 (2012).
Park, J., Gerber, M. H. & Babensee, J. E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A 103, 170–184 (2015).
Shokouhi, B. et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials 31, 5759–5771 (2010).
Rogers, T. H. & Babensee, J. E. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials 32, 1270–1279 (2011).
Ishii, K. J., Coban, C. & Akira, S. Manifold mechanisms of Toll-like receptor-ligand recognition. J. Clin. Immunol. 25, 511–521 (2005).
Bennewitz, N. L. & Babensee, J. E. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials 26, 2991–2999 (2005).
Matzelle, M. M. & Babensee, J. E. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials 25, 295–304 (2004).
Babensee, J. E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 20, 101–108 (2008).
Ng, S. Y. et al. Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer. Radiother. Oncol. 121, 252–257 (2016).
Marabelle, A. et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 123, 2447–2463 (2013).
Fransen, M. F. et al. Effectiveness of slow-release systems in CD40 agonistic antibody immunotherapy of cancer. Vaccine 32, 1654–1660 (2014).
Fransen, M. F., Sluijter, M., Morreau, H., Arens, R. & Melief, C. J. M. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin. Cancer Res. 17, 2270–2280 (2011).
Kaminski, J. M. et al. The controversial abscopal effect. Cancer Treatment Rev. 31, 159–172 (2005).
Gameiro, S. R. et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 5, 403–416 (2014).
Lookingbill, D. P., Spangler, N. & Sexton, F. M. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 22, 19–26 (1990).
Krathen, R. A., Orengo, I. F. & Rosen, T. Cutaneous metastasis: a meta-analysis of data. South. Med. J. 96, 164–167 (2003).
Mehlen, P. & Puisieux, A. Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458 (2006).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Ahmad, A. Introduction to Cancer Metastasis 1st edn (Academic Press, 2016).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2016).
Cormack, R. A., Sridhar, S., Suh, W. W., D'Amico, A. V. & Makrigiorgos, G. M. Biological in situ dose painting for image-guided radiation therapy using drug-loaded implantable devices. Int. J. Radiat. Oncol. 76, 615–623 (2010).
Kumar, R. et al. Nanoparticle-based brachytherapy spacers for delivery of localized combined chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 91, 393–400 (2015).
Stuart, M. A. et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 (2010).
Tada, D. B. et al. Chitosan film containing poly(D,L-lactic-co-glycolic acid) nanoparticles: a platform for localized dual-drug release. Pharm. Res. 27, 1738–1745 (2010).
Acknowledgements
This work was supported by funding from the Brigham and Women's Hospital (BWH) Biomedical Research Institute and the US National Institutes of Health (NIH) grant CA205094-01A1.
Author information
Authors and Affiliations
Contributions
W.N. researched data for the article, made substantial contributions to discussions of the content and wrote the article. O.C.I. researched data for the article, wrote the article and reviewed and/or edited the manuscript before submission. J.D.S., J.H., S.D. and S.C.F. made substantial contributions to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ngwa, W., Irabor, O., Schoenfeld, J. et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18, 313–322 (2018). https://doi.org/10.1038/nrc.2018.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2018.6
This article is cited by
-
Hyperpolarized [1-13C]-pyruvate MRS evaluates immune potential and predicts response to radiotherapy in cervical cancer
European Radiology Experimental (2024)
-
Pyroptotic cell death: an emerging therapeutic opportunity for radiotherapy
Cell Death Discovery (2024)
-
Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment
Nature Communications (2024)
-
In vivo ultrasound-induced luminescence molecular imaging
Nature Photonics (2024)
-
Safety and efficacy of radiotherapy combined with chemotherapy for recurrent metastatic renal pelvic and ureteral carcinoma
World Journal of Urology (2024)