In the Photonics Lab
The impact of optical imaging in biomolecular sciences and medical diagnostics is deeply rooted in our understanding of classical electromagnetism and optics. Our fascination with light goes back centuries, even as early as the Neolithic era, when solar emissions were used to measure (time) and predict (seasonal) events. Isaac Newton first described how white light refracts when it crosses a new medium, where the incident beam angle and the refractive index of the media define the qualitative properties with which light is effectively bent. Thomas Young, a physician and polymath, was the first to experimentally prove that light propagates as a wave. Young’s wave theory of light is the pillar for the subsequent works of Hermann von Helmholtz in optics and of James Clerk Maxwell, whose partial differential equations approximate light-matter interactions and derive the absolute speed of light (which in turn is a cornerstone parameter of general relativity).
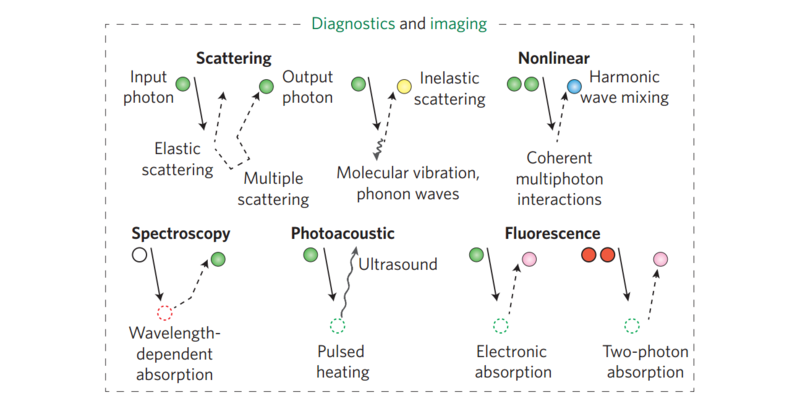
Today’s optical diagnostic systems all adopt these principles. Ultimately, optical diagnostic imaging is based on light: (i) entering a tissue, using a source such as a laser, (ii) interacting with it, typically by diffraction, refraction, scattering and/or absorption, and (iii) being collected via optics or electro-optical sensors.
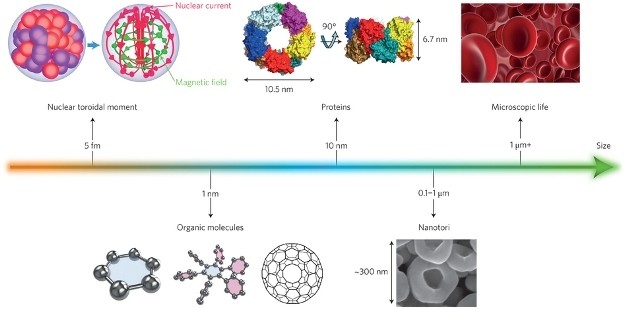
Harnessing light in living tissue, such as neural circuitry, therefore relies on controlling at least some of the dynamical properties of the electromagnetic waves. Recent research has focussed, for example, on understanding the spin-Hall effect and the spin-orbit interactions of light, as well as toroidal dipole electromagnetic excitations in metamaterials.
These metamaterials are of particular interest in imaging, not least for their potential to engineer new tools, such as that of a miniaturised camera, measuring just 1.6 mm × 1.6 mm × 1.7 mm, and capable of producing wide-angle images at near-diffraction-limited resolution. The ability to modify the optical field, known as wavefront-shaping, before it enters a scattering medium has further enabled the generation of micrometre-scale focal spots inside tissue.
Such technologies push the limits of optical imaging towards single-molecule detection, where both labelled (such as fluorescent) and label-free (such as Raman spectroscopy) techniques can be coupled to microscopes. The electron microscope has also benefited from the combined use of coherent light sources and fluorescence emissions for the detection of molecules at the nanometre scale. The observation of dynamic events of single molecules at such resolutions can be achieved using super-resolution microscopy, a technique that is playing a major role in discovering, for example, how our genome is capable of organizing itself in architecturally active chromatin structures or how protein chaperone nanomachines are assembled.