Opinion: DNA-damaging autoantibodies and cancer: the lupus butterfly theory
Philip W. Noble, Sasha Bernatsky, Ann E. Clarke, David A. Isenberg, Rosalind Ramsey-Goldman & James E. Hansen
Nature Reviews Rheumatology
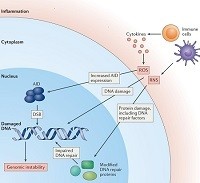 A subset of lupus autoantibodies can penetrate nuclei and damage DNA in cells, with consequences for the pathophysiology of systemic lupus erythematosus as well as cancer risk. Noble et al. propose the lupus butterfly theory to explain the effects of these DNA-damaging lupus autoantibodies on the interplay between autoimmunity, DNA damage and cancer.
A subset of lupus autoantibodies can penetrate nuclei and damage DNA in cells, with consequences for the pathophysiology of systemic lupus erythematosus as well as cancer risk. Noble et al. propose the lupus butterfly theory to explain the effects of these DNA-damaging lupus autoantibodies on the interplay between autoimmunity, DNA damage and cancer.
Timeline: The evolution of drug discovery in systemic lupus erythematosus
Daniel J. Wallace
Nature Reviews Rheumatology
Drug discovery in systemic lupus erythematosus (SLE) has lagged behind other rheumatic diseases, in large part because of difficulty in measuring change or improvement in a disorder that involves multiple organ systems to varying degrees at different times. The metrics currently used as primary endpoints are composite indices that rely mainly on disease assessment measures derived before the era of clinical trials of targeted therapies. Only one agent has been approved for the treatment of SLE since 1957. This monograph reviews the evolution of drug development for SLE, problems and pitfalls that have been encountered, and outlines the domains used to evaluate SLE in the clinic. Finally, several initiatives underway to improve clinical trial design are outlined.
Opinion: The BAFFling effects of rituximab in lupus: danger ahead?
Michael R. Ehrenstein & Charlotte Wing
Nature Reviews Rheumatology
 Ehrenstein and Wing assert that rituximab re-treatment can trigger a vicious circle of ever-rising levels of BAFF (B-cell-activating factor), increasing autoantibody production and worsening disease in some patients with SLE. They argue for combining B-cell depletion and BAFF blockade in patients with SLE who have post-rituximab flares characterized by high levels of antibodies to double-stranded DNA.
Ehrenstein and Wing assert that rituximab re-treatment can trigger a vicious circle of ever-rising levels of BAFF (B-cell-activating factor), increasing autoantibody production and worsening disease in some patients with SLE. They argue for combining B-cell depletion and BAFF blockade in patients with SLE who have post-rituximab flares characterized by high levels of antibodies to double-stranded DNA.
Systemic lupus erythematosus in 2016: Gene expression profiling comes closer to the clinic
Guillermo Barturen & Marta E. Alarcón-Riquelme
Nature Reviews Rheumatology
Gene expression profiling has been used for the first time to stratify patients with systemic lupus erythematosus (SLE) into potentially useful clinical groups, and also to further understand differences in the cell-specificity and nature of the interferon signature typical of SLE and other autoimmune diseases.
Systemic lupus erythematosus: Extent and patterns of off-label use of rituximab for SLE
Iñaki Sanz
Nature Reviews Rheumatology
Despite conflicting evidence from clinical trials, rituximab continues to be used off-label in the treatment of systemic lupus erythematosus (SLE). A new study has now investigated the use of this drug for SLE in Europe, including indications for use and patient characteristics.
Connective tissue diseases: Promises and challenges of metabolomics in SLE
Huihua Ding & Chandra Mohan
Nature Reviews Rheumatology
Despite a multitude of confounding variables, common themes are emerging in metabolomic studies of systemic lupus erythematosus (SLE). Newly revealed metabolites and pathways are likely to complement the biomarkers and insights into SLE pathogenesis that are emerging from genomic, transcriptomic and proteomic studies.
Lupus nephritis: MAINTAINing perspective in lupus nephritis trials
Brad H. Rovin & Isabelle Ayoub
Nature Reviews Nephrology
Despite aggressive therapy, lupus nephritis (LN) remains an important predictor of morbidity in patients with systemic lupus erythematosus. Clinical trials of novel drugs have not improved LN outcomes; however, re-analysis of well-characterized cohorts has identified surrogate end points of long-term renal survival, which will facilitate testing and qualification of novel treatments.
Autoimmunity: Do IgM antibodies protect against atherosclerosis in SLE?
Maureen McMahon & Brian Skaggs
Nature Reviews Rheumatology
Autoantibodies against end products of lipid peroxidation have unexpected effects on atherosclerosis in systemic lupus erythematosus (SLE). The discovery of their effects could lead to new theories about the role of the adaptive immune system in SLE.
Clinical trials: The rise of IL-2 therapy — a novel biologic treatment for SLE
Jens Y. Humrich & Gabriela Riemekasten
Nature Reviews Rheumatology
An unmet need exists for more-effective and selective therapeutics in systemic lupus erythematosus. Advances in understanding of the pathogenetic mechanisms of this severe autoimmune disease have led to the clinical translation of low-dose IL-2 therapy, which primarily aims to restore the activity of regulatory T cells.
Connective tissue diseases: Targeting type I interferon in systemic lupus erythematosus
Timothy B. Niewold
Nature Reviews Rheumatology
 Type I interferon (IFN) is an attractive therapeutic target in systemic lupus erythematosus (SLE), a notion bolstered by the positive results of a recent clinical trial of the anti-IFN antibody sifalimumab in patients with SLE. Interestingly, sifalimumab worked best in patients with high expression of IFN-induced genes, supporting the idea that subgroups of patients might be targeted specifically.
Type I interferon (IFN) is an attractive therapeutic target in systemic lupus erythematosus (SLE), a notion bolstered by the positive results of a recent clinical trial of the anti-IFN antibody sifalimumab in patients with SLE. Interestingly, sifalimumab worked best in patients with high expression of IFN-induced genes, supporting the idea that subgroups of patients might be targeted specifically.
Connective tissue diseases: Mitochondria drive NETosis and inflammation in SLE
Eric Boilard & Paul R. Fortin
Nature Reviews Rheumatology
 Mitochondria are the powerhouses of the cell, providing energy through oxidative respiration. Possibly owing to their similarities with bacteria, however, mitochondria extruded from cells promote inflammation. New research demonstrates that in systemic lupus erythematosus, mitochondrial respiration is critical in neutrophil extracellular trap formation, and that mitochondria released by neutrophils induce inflammatory cytokine production.
Mitochondria are the powerhouses of the cell, providing energy through oxidative respiration. Possibly owing to their similarities with bacteria, however, mitochondria extruded from cells promote inflammation. New research demonstrates that in systemic lupus erythematosus, mitochondrial respiration is critical in neutrophil extracellular trap formation, and that mitochondria released by neutrophils induce inflammatory cytokine production.
Autoimmunity: Interferon α or β: which is the culprit in autoimmune disease?
Mary K. Crow
Nature Reviews Rheumatology
Type I interferons contribute to the pathogenesis of several autoimmune diseases, with each interferon being preferentially produced in different settings by different cells. Could knowing which one is predominant in a given disease — or patient — lead to better therapies?
Glomerular disease: Personalized immunomonitoring in lupus and lupus nephritis
Hans-Joachim Anders & Matthias Kretzler
Nature Reviews Nephrology
The heterogeneity of pathomechanisms leading to systemic lupus erythematosus (SLE) might contribute to between-patient variations in treatment response. A new, longitudinal transcriptome analysis has identified molecularly distinct subgroups of SLE that correlate with disease activity; use of such disease classifiers might facilitate the development of stratified treatment recommendations.
Connective tissue diseases: Remission in SLE — are we there yet?
Eric F. Morand
Nature Reviews Rheumatology
 The concept of treating to a target of remission is gaining ground in systemic lupus erythematosus. New research suggests that achievement of this treatment goal is rare, but are the definitions of remission used in these studies fit for purpose?
The concept of treating to a target of remission is gaining ground in systemic lupus erythematosus. New research suggests that achievement of this treatment goal is rare, but are the definitions of remission used in these studies fit for purpose?
Connective tissue diseases: Is SLE many single-organ diseases or an overlapping spectrum?
Joan T. Merrill
Nature Reviews Rheumatology
Emerging science characterizes lupus as a systemic spectrum disorder, rather than as multiple single-organ diseases. So is it time to capitalize on this progress and begin evaluating treatment options on the basis of physiologic mechanisms instead of organ involvement?

