Abstract
Both clinical and experimental evidence have firmly established that chronic pancreatitis, in particular in the context of Kras oncogenic mutations, predisposes to pancreatic ductal adenocarcinoma (PDAC). However, the repertoire of molecular mediators of pancreatitis involved in Kras-mediated initiation of pancreatic carcinogenesis remains to be fully defined. In this study we demonstrate a novel role for vacuole membrane protein 1 (VMP1), a pancreatitis-associated protein critical for inducible autophagy, in the regulation of Kras-induced PDAC initiation. Using a newly developed genetically engineered model, we demonstrate that VMP1 increases the ability of Kras to give rise to preneoplastic lesions, pancreatic intraepithelial neoplasias (PanINs). This promoting effect of VMP1 on PanIN formation is due, at least in part, by an increase in cell proliferation combined with a decrease in apoptosis. Using chloroquine, an inhibitor of autophagy, we show that this drug antagonizes the effect of VMP1 on PanIN formation. Thus, we conclude that VMP1-mediated autophagy cooperate with Kras to promote PDAC initiation. These findings are of significant medical relevance, molecules targeting autophagy are currently being tested along chemotherapeutic agents to treat PDAC and other tumors in human trials.
Similar content being viewed by others
Main
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths in the Western world and predicted to be the second one in 2030.1 The initiation, progression, and maintenance of PDAC results from the interplay of genetic events combined with other multiples less well-characterized factors.2 The genetic alterations contributing to PDAC pathogenesis have been extensively studied and clearly determined. Among the most common genetic alterations contributing to pancreatic carcinogenesis, oncogenic mutations in KRAS are the most frequently detected not only in frank PDAC but also in its best characterized preneoplastic disease, namely chronic pancreatitis. Oncogenic KRAS signals initiate acinar-to-ductal metaplasia, a step essential for the formation of preneoplastic lesions, that together with mutations in tumor suppressors such as CDKN2A, TP53, and SMAD4 occurring during the progression from pre-neoplastic pancreatic intraepithelial neoplasia (PanIN) lesions, promotes the development of invasive cancer.3 Thus, oncogenic mutations in KRAS are necessary to initiate cancer formation and as such remain one of the most studied genetic alterations in PDAC. However, the entire repertoire of pathways contributing with this phenomenon remains elusive.
Autophagy has been proposed as a cellular contributing to pancreatic carcinogenesis and in particular the tumor initiating effects of KRAS.4, 5, 6, 7 Indeed, oncogenic KRAS generates a metabolic stress characterized by a temporary deficit in energy which must be compensated by increasing metabolic resources through the activation of autophagy.4, 5, 6, 7 However, the role of autophagy as pro- or anti-tumor is largely debated because it seems to be conditioned by the pathway regulating this phenomenon, the genomic status of the transforming cell population as well as both the physiological and pathological context in which this process is activated.8, 9 Consequently, more work is needed to define the repertoire of autophagy mediators, and pathways, which either promote or antagonize PDAC development. Thus, autophagy mediators, which also work in pancreatitis, are good candidates as modifiers of the effect of oncogenic pathways leading to pancreatic transformation.
We have previously identified a pancreatitis-induced transmembrane protein known as vacuole membrane protein 1 (VMP1), which regulates an inducible form of autophagy.10, 11 Mechanistically, VMP1 is involved in the phagophore formation by directly binding to beclin1.11 Noteworthy, VMP1 expression is transcriptional induced by oncogenic KRAS via a GLI3-p300-dependent mechanism.12 Therefore, VMP1 is strongly induced by two complementary PDAC-promoting pathways, namely, pancreatitis and activated KRAS, which further support the hypothesis that this protein may be necessary to initiate neoplastic transformation. To test this hypothesis, we developed a novel mice model in which the VMP1 is induced specifically in the pancreas by doxycycline together with activation of the oncogenic KrasG12D. This model allowed us to evaluate the effects of VMP1 on PDAC initiation as well as serve as a platform for preclinical trials, which can evaluate the role of autophagy inhibitors on PanIN development. The results of these experiments support the hypothesis stated above and unravel, for the first time, a new role for VMP1-mediated autophagy in the promotion of KRAS-mediated PDAC initiation. Moreover, through a preclinical trial that uses chloroquine to inhibit autophagy we demonstrate that the promoting effects of VMP1 on initiation can be reversed. Thus, combined, these results reinforce the idea that distinct pancreatitis-associated pathways, in particular those that regulate autophagy, have the ability to contribute to the process of pancreatic carcinogenesis. Lastly, our findings further underscore the potential utility of inhibiting autophagy to inhibit PDAC growth.
Results
Development of the Pdx1-cre-KrasG12D/VMP1DsRed mice, a novel genetically engineered mouse model for studying the role of inducible autophagy on PDAC initiation
The VMP1 protein has recently elicited attention because it is a key regulator of autophagy in organisms ranging from Caenorhabditis elegans to mammals.11, 13, 14 As it regards to the pancreas, recent studies have demonstrated that when fused to green fluorescent protein, the transgenic expression of VMP1 in the exocrine cells of the murine gland, this protein increases the levels of autophagy.11, 15 In the current study, however, we sought to define the role of VMP1 in the regulation of PDAC initiation using a mechanistic design based on expressing VMP1 in murine pancreatic cells by creating a novel genetically engineered mouse model (GEMM) co-expressing VMP1-DsRed and oncogenic KrasG12D in the exocrine pancreas. Because VMP1 is a regulator of ‘inducible autophagy’ and this process can have either a positive or negative impact on the regulation of pancreatic cell growth depending upon the cellular context, we designed this GEMM to express a doxycycline inducible VMP1-DsRed protein from a bi-cistronic cassette that also contains an IRES-luciferase sequence (Figure 1). When crossed to Pdx1-Cre-‘Tet on’ mouse, this animal allows us to control for the expression of the transgene by both fluorescence and luciferase assays. Indeed, Figure 1 shows that upon induction with doxycycline, these mice readily express both VMP1DsRed and luciferase. Notably, VMP1DsRed is induced as punctate in the pancreas of animals treated with doxycycline concomitantly with the relocalization of LC3 as dots (Figure 1). Thus, together, these results demonstrate that our novel genetically engineered mouse readily express VMP1 in an inducible and tractable manner (see Supplementary Figure 1), suggesting that it can become a useful tool for better understanding the role of this protein during PDAC initiation.
Construct Ptight-VMP1DsRed-IRES-Luciferase Renilla-SV40 polyA is functional in vitro and in vivo. VMP1DsRed-IRES-Luciferase Renilla construct was transfected in HEK293 cells and 24 h later treated with 500 ng/ml doxycycline and 24 h later DsRed expression was observed (a). Luciferase activity measured after 24 h of treatments with 100, 500, and 1000 ng/ml doxycycline (*P<0.05, **P>0.01, and ***P>0.001) mean±S.E.M (b). Expression of the VMP1DsRed (c) and Luciferase activity (d) in the pancreas of the transgenic mice treated or not with doxycycline dissolved in drinking water was measured by Western blot using a VMP1 antibody. Loading was controlled by using a β-Tubulin antibody. LC3 cleavage was measured by Western blot (e). Staining of VMP1dsRed and LC3 by IHC (f). VMP1dsRed and LC3-II isoform was more abundant in the pancreas of transgenic mice treated with doxycycline than in controls. IHC reveals punctate distribution in the pancreas of transgenic mice treated with doxycycline whereas it is not detected (VMP1dsRed) or homogeneously distributed in the cytoplasm (LC3) of controls animals. Scale bars=50 μm
Inducible expression of VMP1 promotes KrasG12D-mediated PDAC initiation
To define the role of VMP1 in PDCA initiation, we initially determine the levels of PanIN formation in the doxycycline-treated Pdx1-cre;KrasG12D;VMP1DsRed mice (Figure 2). As expected control mice sacrificed at 14 and 20 weeks old of age control mice developed extensive ADM as wells as low and high-grade PanINs (average of PanIN per field was 19±5, n=6, and 26±6, n=5, P<0.05). In contrast, animals in which VMP1DsRed expression was induced by doxycycline revealed an increased number of PanINs (average of PanIN per field was 38±10 (n=6), and 47±13 (n=5, P<0.05). Congruent with this result, Figure 3 shows that VMP1DsRed-expressing pancreatic cells exhibited a significant increase in proliferating cells as demonstrated by higher levels of Ki67 positive staining (43±14 versus 102±9 at 14 weeks old and 47±9 versus 111±12 at 20 weeks old, P<0.05). In addition, Figure 4 shows that VMP1DsRed-expressing animals have a decreased in the numbers of apoptotic cells as demonstrated by activated caspase 3 staining (132±11 versus 31±8 at 14 weeks old and 159±16 versus 37±14 at 20 weeks old, P<0.001). As a control we treated Pdx1-cre-KrasG12D mice with doxycycline and found not significant differences in PanIN development. Average of PanIN per field was 21±7 in doxycycline-treated mice (n=3), and 18±5 for their control (n=3). Combined, these results demonstrate, for the first time, that VMP1 promotes PDAC initiation by oncogenic Kras via a mechanism stimulating cell survival and proliferation.
PanINs development is increased in Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice. (a) Representative pictures of pancreas from Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice stained with Alcian blue at 14 and 20 weeks old treated with doxycycline dissolved in drinking water. (b) Number of PanIN lesions per × 20 tissue field in Pdx1-cre; LSL-KrasG12D; VMP1DsRed treated with doxycycline or control at 14 and 20 weeks of age. (*P<0.05 and **P>0.01) mean±S.E.M. Scale bars=50 μm
KI67 staining is increased in the pancreas of Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice treated with doxycycline. Immunostaining of the Ki67 antigen was performed to estimate the proliferation activity in the pancreas of Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice treated or not with doxycycline. (a) Representative pictures of pancreas from Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice stained with anti-Ki-67 at 14 and 20 weeks old treated with doxycycline dissolved in drinking water. (b) Quantification of positive nuclei per × 20 tissue field in Pdx1-cre;LSL-KrasG12D;VMP1DsRed treated with doxycycline or control at 14 and 20 weeks of age. (*P<0.05 and NS corresponds to not significant) mean±S.E.M. Scale bars=50 μm
Activity of caspase 3 is decreased in the pancreas of Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice treated with doxycycline. Immunostaining with the antibody M30 was performed to estimate the caspase 3 activity in the pancreas of Pdx1-cre;LSL-KrasG12D; VMP1DsRed mice treated or not with doxycycline. (a) Representative pictures of pancreas from Pdx1-cre; LSL-KrasG12D; VMP1DsRed mice stained with the M30 antibody at 14 and 20 weeks old treated with doxycycline dissolved in drinking water. (b) Quantification of positive cells per × 20 tissue field in Pdx1-cre;LSL-KrasG12D;VMP1DsRed treated with doxycycline or control at 14 and 20 weeks of age. (*P<0.05, **P<0.01, ***P<0.001, and NS corresponds to not significant) mean±S.E.M. Scale bars=50 μm
VMP1 promotes KrasG12D-mediated PDAC initiation through the regulation of autophagy
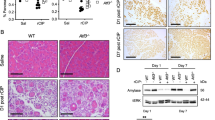
We next evaluated the effects of inducing the VMP1DsRed protein on autophagy by measuring LC3 cleavage, a well established marker of autophagy. As previously reported by our group and others, the induction of VMP1 promotes cleavage of LC3-II by 7.4±3.1 folds, P<0.05 (Figure 1). Congruent with these results, immunohistochemistry (IHC) analysis reveals that the VMP1DsRed signal adopts a punctate distribution pattern, reminiscent to the localization of proteins that mediates cell autophagy (Figure 1). To gain insight as whether the effects of VMP1 in promoting PanIN formation was mediated through its autophagic function, we treated these animals, daily, with chloroquine (60 mg/kg), starting at 3 weeks of age, a period that coincides with a robust initiation of PanIN formation. As a control, we measured the amount of LC3 cleavage in liver. Figure 5 shows that, indeed after this period of treatment, liver cells display a significant accumulation of the LC3-II isoform, revealing that this drug efficiently blocks autophagic flux in vivo. We then evaluated the effect of chloroquine on PanIN development in both 14 and 20 weeks old Pdx1-cre; KrasG12D;VMP1DsRed mice treated with doxycycline. The results of these experiments are shown in Figure 5. Control animal developed an average of 4.8±1.8, (n=4) PanIN lesions at 14 weeks and 7.5±1.9 (n=4) at 20 weeks. As expected, the number of PanIN lesions was significantly increased in mice treated with doxycycline (29.8±4.5 (n=4) and 41.1±5.5 (n=4) at 14 and 29 weeks, respectively, P>0.05) but significantly reduced in these animals when treated with chloroquine with an average of 6.2±2.1, (n=4), P<0.05 and 11.0±5.2, PanINs (n=4), P<0.05 at 14 and 20 week old, respectively). Thus, we conclude that the effect of VMP1 in promoting PDAC initiation by oncogenic Kras is medicated through its effects on autophagy.
PanINs development is inhibited by the concomitant treatment with chloroquine in Pdx1-cre;LSL-KrasG12D;VMP1DsRed mice. (a) Representative pictures of pancreas from Pdx1-cre; LSL-KrasG12D; VMP1DsRed mice stained with Alcian blue at 14 and 20 weeks old treated with or without doxycycline dissolved in drinking water concomitantly with choroquine or not. (b) Number of PanIN lesions per × 20 tissue field in Pdx1-cre; LSL-KrasG12D; VMP1DsRed treated with doxycycline or not together or not with chloroquine at 14 and 20 weeks of age. (*P<0.05 and NS corresponds to not significant) mean±S.E.M. Scale bars=50 μm. (c) Efficiency of the chloroquine treatment was controlled in liver of these mice by measuring the cleavage of LC3 protein by western blot with an anti LC3 antibody
In summary, at the onset of this study, the role of VMP1 in pancreatitis has been established.15 However, nothing was known about the effects of VMP1-mediated autophagy on oncogenic Kras-mediated PDAC initiation. Addressing this question became of significant importance since pancreatitis predisposes to cancers but the molecules, which are candidates to mediate this transition, remain a topic of extensive investigation. Our research led to the generation of a new model in which the role of VMP1-mediated autophagy on aspects related to pancreatic carcinogenesis can be reliably study. Using this model, we find that VMP1 exerts a promoting effect on PDAC initiation by mediating the formation of PanIN through an increased in cell proliferation and a decrease in cell death by apoptosis. In addition, we demonstrate that chloroquine, a drug currently being tested in clinical trials for the therapeutics of PDACs, reverse this effect. Thus, combined these results bear both mechanistic and biomedical relevance for better understanding and potentially targeting pathways which are critical for initiating pancreatic carcinogenesis.
Discussion
Even though the role of pancreatitis as a promoting stimuli for the initiation of PDAC has been extensively documented for both human and mouse, the molecular mediators of this phenomenon remain to be fully characterized. Consequently, the current study was designed to begin filling this gap in knowledge by studying how the pancreatitis-associated protein VMP1, a key regulator of autophagy, cooperates with oncogenic KRAS to give rise to PanINs. This is also important since the role of autophagy in PDAC development remains a controversy. Some studies, for instance, have reported that autophagy promotes cancer development whereas others have demonstrated the opposite effect. Our experimental design involved the development of a new mouse model in which the oncogene Kras was activated in the pancreas by the expression of the Pdx1-cre while VMP1 was simultaneously induced by doxycycline. We observed that expression of the VMP1 is accompanied by a significant increase in the number of PanIN lesions and the concomitant treatment with chloroquine abolishes this effect. We concluded that the promoting effect of VMP1 on KRAS-induced PDAC initiation is mediated through its role as a regulator of autophagy. Thus, this information extends the repertoire of pancreatitis-associated proteins as well as autophagy regulators, which can contribute to pancreatic carcinogenesis.16, 17, 18
In light of these results it becomes important to review and discuss the known functions of VMP1. For instance, it has been established that this protein is involved in the initiation of autophagy since, cells lacking VMP1 have elevated and aberrant PtdIns3P signaling on the ER, resulting in an increased and persistent recruitment of Atg18 and other autophagic proteins.14 In addition, although ULK1 and ATG5 are separated in the genetic hierarchy, these proteins synchronously accumulate at preexisting VMP1-positive punctate structures, followed by recruitment of ATG14, ZFYVE1, and WIPI1.19 Moreover, VMP1 bind directly to the BH3 motif of beclin 1 leading to the formation of a complex with the Class III phosphatidylinositol-3 kinase hVps34, a key positive regulator of autophagy, at the site where autophagosomes are generated. Interestingly, the interaction between beclin 1 and VMP1 leads to the dissociation of Bcl-2 from beclin 1 increasing the intracellular amount of the available beclin 1 to induce autophagy.20 Furthermore, VMP1 regulates autophagosome formation by shortening the duration of omegasomes and therefore accelerating the autophagy flux.13 Lastly, in Dictyostelium, VMP1-inactivated mutant cells showed accumulation of massive ubiquitin-positive protein aggregates containing the autophagy marker Atg8 and p62 homologue demonstrating that VMP1 is required for the clearance of these ubiquitinated protein aggregates through autophagy.21 Thus, VMP1 is both, a regulator of autophagy during pancreatitis since its overexpression activates signaling to promote autophagosome formation and also a transmembrane protein integrated into the autophagosome. However, until the current study was completed nothing was known about the role of VMP1-mediated autophagy and pancreatic carcinogenesis. This observation is of significant importance since the role of autophagy in cancer development is multiple and complex. In fact, a plethora of studies indicate that autophagy may either favor or constrain tumor development and progression depending of the intracellular pathways considered. For this reason, it is difficult to predict as whether a particular regulator of autophagy would have a positive or negative effect on neoplastic transformation making the type of experimentation reported in this study a ‘sine qua non’ effort.
An important question in the field of pancreatology is how pancreatitis promotes the formation of PanINs.22 An insight into this question is provided by studies, which show that during pancreatitis autophagy is systematically activated, frequently as a protective function, to avoid the development or help in the recovery phase of the disease.23, 24 It is likely that, at least part of the protective effect of autophagy is related to both its role in the sequestration of zymogen granules that contain the deleterious enzymes that kill acinar cells and by supporting the enhanced metabolic rate that accompany cell growth during regeneration.15 We therefore hypothesized that, in the presence of oncogenically active KRAS, autophagy as induced by pancreatitis-associated proteins such as VMP1 may serve at least as one of the molecules that link this disease with the process of cancer initiation. In fact, the data described as result of the current study support this idea.
It is also important to discuss the role that autophagy plays in cancer development, as it appears to be varied and complex. In fact, it has been previously demonstrated that autophagy mediates the mitotic senescence transition.25 Autophagy is activated upon acute induction of this cellular process to facilitate the senescence associated secretory phenotype, which also creates an inflammatory microenvironment.26 Senescence is known to be a major anticancer pathway that is activated in response to the oncogenic Kras activation and, in this context therefore, senescence enhanced by autophagy could help to inhibit the oncogenic effect induced by KrasG12D. In addition, autophagy activation acts as an antiapoptotic factor is some tissues, depending of the biological circumstances.27, 28 Moreover, following its oncogenic activation, Kras induces a metabolic stress due to the increased metabolic needs of the cell that can be bypassed by activating the autophagy as a source of energy.29, 30 All these above mentioned mechanisms may modulate, favor, or even antagonize tumor development and progression depending of the intracellular pathways, which is operational at a particular state of the cell carrying Kras mutations, and may likely explain the often contrasting results previously reported on the role of autophagy in cancer.
Similarly important is to consider that autophagy is suspected to be a mediator of cancer resistance to both radiotherapy and chemotherapy with certain drugs.31, 32 However, the mechanisms by which autophagy acts in cancer resistance is also complex since anticancer treatments often damage intracellular organelles thereby creating protein aggregates that must be cleared by autophagy is to protect cells. On the other hand, autophagy has also been reported to act as a mediator of chemotherapy-induced cell death in cancer.33 The mechanism of action of the autophagy-dependent cell death is not clearly established although it seems to be mediated throughout the caspase 3 activation.33 Therefore, just as we discussed for the role of autophagy in cancer initiation and progression, reports supporting or contradicting its function in chemotherapeutics are abundant in the literature.34, 35 Nevertheless, the fact that cancer treatment induces autophagy systematically is currently unambiguous.36 Thus, congruent with this knowledge, co-treatment with the autophagy inhibitor chloroquine improves the effect of a significant number of anticancer drugs.37, 38, 39, 40, 41
In conclusion, we report that VMP1 promotes PanIN development in the presence of an oncogenic Kras signaling pathway activation through its role in autophagy.
Materials and Methods
Mice
We developed a DNA construct named Ptight-VMP1DsRed-IRES-Luciferase Renilla-SV40 polyA by subcloning the cDNA encoding VMP1-DsRed fusion proteins into the EcoRI/NheI restriction sites and the cDNA encoding luciferase renilla into the BamHI/EcoRV restriction sites of the pTRE-Dual1 plasmid (Clontech Laboratories Inc., Palo Alto, CA, USA). The construct was sequenced and its response to the doxycycline evaluated on HEK293T cells. The transgene was separated from plasmid vector sequences by XhoI digestion and purified after agarose gel electrophoresis with Elutip-d columns (Schleicher & Schuell, Dassel, Germany) and ethanol precipitation. Microinjections into fertilized oocytes derived from B6D2 mice were realized at the Service d’Expérimentation Animale et Transgenèse (SEAT, CNRS, Villejuif, France). Founders were identified by polymerase chain reaction (PCR) analyses of tail DNAs from 2-week-old mice using oligonucleotides specific to the DsRed-GTFw (5′-CTTCAAGACCGTGTACAAGGCCAAG-3′) and IRES-GTRev (5′-GGGTCGCTACAGACGTTGTTTGTCTTC-3′) and RlucFw (5′-TTTTTGGATCCATGACCAGCAAGGTGTACGACCC-3′) and RlucRev (5′-TTTTGATATCCTGCTCGTTCTTCAGCACTCTCTCCACG-3′). PCR conditions are available upon request.
Pdx1-cre and LSL-KrasG12D mice strains have been described elsewhere.17, 42 Because animals are from different genetic backgrounds, we systematically used littermate control and experimental mice. Mice were kept within the Experimental Animal House of the Centre de Cancérologie de Marseille (CRCM), pole Luminy, following institutional guidelines.
Doxyclycline Hyclate and Hydroxychloroquine were purchased from Sigma-Aldrich. Transgenic mice were exposed to doxycycline (250 mg/l) dissolved in 5% sucrose supplied as drinking water, which was protected from the light and exchanged every 3 days. Mice received daily intraperitoneal injections of chloroquine (60 mg/kg), or saline, started at the end of 3 weeks old and for 10 or 16 weeks.
Histology and IHC
Pancreatic sections were fixed in 4% paraformaldehyde and paraffin embedded. H&E and Alcian blue staining were performed using standard procedures. Sections were probed with primary antibodies against Ki67 (BioLegend, San Diego, CA) to estimate the proliferation activity, against LC3 (Medical & Biological Laboratories, Nagoya, Japan) to measure autophagosome formation and against the cleaved CK18 to study the apoptotic activity. In fact, during apoptosis, CK18 undergoes dramatic reorganization and is cleaved by the caspases, generating an apoptosis-specific neo-epitope recognized by the monoclonal antibody M30 (Cell Signaling, Beverly, MA). Samples were examined in an Eclipse 90i Nikon microscope.
Quantification of lesions per mouse
Number of lesions per field was counted and the types of lesion were classification on H&E-stained slides. Quantification represents the average of 15–20 × 20 fields of view from 4 to 6 mice of each genotype.
Immunoblotting
Protein extraction was performed on ice using total protein extraction buffer: 50 mM HEPES pH 7.5, 150 mM NaCl, 20% SDS, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton, 25 mM NaF, 10 μM ZnCl2, 50 mM DTT. Before lysis, protease inhibitor cocktail at 1:200 (Sigma-Aldrich, NUPR1340), 500 μM PMSF, 1 mM sodium orthovanadate, and 1 mM β glycerophosphate were added. Protein concentration was measured using a bicinchoninic acid assay protein assay kit (Pierce Biotechnology). Protein samples (60 μg) were denatured at 95 °C and subsequently separated by SDS-PAGE gel electrophoresis. After transfer to nitrocellulose, membrane was blocking with 1% BSA, samples were probed with primary antibody followed by a horseradish peroxidase couple secondary antibody. Primary antibodies against LC3 was from Medical & Biological Laboratories (Nagoya, Japan) and antibody against VMP1 was homemade.11 Polyclonal antibody against β-Tubulin was used as a loading control was from Sigma-Aldrich (St. Louis, MO). Image acquisition was made in Fusion FX image acquisition system (Vilber Lourmat) and bands were quantified ImageJ software (NIH).
RT-qPCR
One μg RNA from pancreas was reversed transcribed using the Go Script reagent (Promega) according to manufacturer’s instructions. Real-time quantitative PCR for VMP1 was performed in a Stratagene cycler using Takara reagents with the following primers sequences 5′-CTGGCAGTTCAAAAACTAGTAC-3′ and 5′-CCGGGGACAGCACCAATGAAAG-3′ which recognize both human and mouse transcripts.
Statistical analysis
One-way variance analysis was used to calculate significances between Pdx1-Cre;KrasG12D;VMP1DsRed and control per tissue (%) as previously described.43 To compare KI67 and caspase 3 activity one-way variance analysis was used, P value was calculated used delta Ct as described by Yuan et al.44 For compared numbers of lesion per field in between groups at 14 and 20 weeks old, a two-way variance analysis was used. Values were expressed as mean±S.E.M. All tests of significance were two-tailed and the level of significance was set at 0.05. All statistics test were performed using IBM SPSS statistics 21.
Abbreviations
- VMP1:
-
vacuole membrane protein 1
- PDAC:
-
pancreatic ductal adenocarcinoma
- PanIN:
-
pancreatic intraepithelial neoplasia
- GFP:
-
green fluorescent protein
- DsRed:
-
red fluorescent protein
- GEMM:
-
genetically engineered mouse model
- IHC:
-
immunohistochemistry
- PCR:
-
polymerase chain reaction
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- EGTA:
-
ethylene glycol tetraacetic acid
- DTT:
-
1,4-dithiothreitol
- BCA:
-
bicinchoninic acid assay
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM . Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 4006.
Iovanna JL, Marks DL, Fernandez-Zapico ME, Urrutia R . Mechanistic insights into self-reinforcing processes driving abnormal histogenesis during the development of pancreatic cancer. Am J Pathol 2013; 182: 1078–1086.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20: 1218–1249.
Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 2011; 25: 717–729.
Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 2011; 25: 460–470.
Kim MJ, Woo SJ, Yoon CH, Lee JS, An S, Choi YH et al. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem 2011; 286: 12924–12932.
Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell 2011; 22: 165–178.
Rosenfeldt MT, O'Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013; 504: 296–300.
Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov 2014; 4: 905–913.
Dusetti NJ, Jiang Y, Vaccaro MI, Tomasini R, Azizi Samir A, Calvo EL et al. Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun 2002; 290: 641–649.
Ropolo A, Grasso D, Pardo R, Sacchetti ML, Archange C, Lo Re A et al. The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem 2007; 282: 37124–37133.
Lo Re AE, Fernandez-Barrena MG, Almada LL, Mills LD, Elsawa SF, Lund G et al. Novel AKT1-GLI3-VMP1 pathway mediates KRAS oncogene-induced autophagy in cancer cells. J Biol Chem 2012; 287: 25325–25334.
Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010; 141: 1042–1055.
Calvo-Garrido J, King JS, Munoz-Braceras S, Escalante R . Vmp1 regulates PtdIns3P signaling during autophagosome formation in Dictyostelium discoideum. Traffic 2014; 15: 1235–1246.
Grasso D, Ropolo A, Lo Re A, Boggio V, Molejon MI, Iovanna JL et al. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem 2011; 286: 8308–8324.
Baumgart S, Chen NM, Siveke JT, Konig A, Zhang JS, Singh SK et al. Inflammation-induced NFATc1-STAT3 transcription complex promotes pancreatic cancer initiation by KrasG12D. Cancer Discov 2014; 4: 688–701.
Hamidi T, Algul H, Cano CE, Sandi MJ, Molejon MI, Riemann M et al. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest 2012; 122: 2092–2103.
Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI et al. IL17 functions through the novel REG3beta-JAK2-STAT3 inflammatory pathway to promote the transition from chronic pancreatitis to pancreatic cancer. Cancer Res 2015; 75: 4852–4862.
Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N . Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy 2013; 9: 1491–1499.
Molejon MI, Ropolo A, Re AL, Boggio V, Vaccaro MI . The VMP1-Beclin 1 interaction regulates autophagy induction. Sci Rep 2013; 3: 1055.
Calvo-Garrido J, Escalante R . Autophagy dysfunction and ubiquitin-positive protein aggregates in Dictyostelium cells lacking Vmp1. Autophagy 2010; 6: 100–109.
Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007; 11: 291–302.
Diakopoulos KN, Lesina M, Wormann S, Song L, Aichler M, Schild L et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology 2015; 148: 626–638 e617.
Gukovsky I, Gukovskaya AS . Impaired autophagy underlies key pathological responses of acute pancreatitis. Autophagy 2010; 6: 428–429.
Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF et al. Autophagy mediates the mitotic senescence transition. Genes Dev 2009; 23: 798–803.
Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 2011; 332: 966–970.
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G . Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 2014; 15: 81–94.
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005; 122: 927–939.
White E . Exploiting the bad eating habits of Ras-driven cancers. Genes Dev 2013; 27: 2065–2071.
Mathew R, Autophagy White E . Stress, and cancer metabolism: what doesn't kill you makes you stronger. Cold Spring Harb Symp Quant Biol 2011; 76: 389–396.
Yang MC, Wang HC, Hou YC, Tung HL, Chiu TJ, Shan YS . Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol Cancer 2015; 14: 179.
Ding L, Ma G, Liu Y, Jia Y, Liu X . Autophagy blockage enhances radiosensitivity of osteosarcoma MG-63 cells in vitro. Clin Lab 2015; 61: 1365–1372.
Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 2009; 119: 1359–1372.
Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK et al. The autophagic paradox in cancer therapy. Oncogene 2012; 31: 939–953.
Grander D, Panaretakis T . Autophagy: cancer therapy's friend or foe? Future Med Chem 2010; 2: 285–297.
Kondo Y, Kanzawa T, Sawaya R, Kondo S . The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005; 5: 726–734.
Lefort S, Joffre C, Kieffer Y, Givel AM, Bourachot B, Zago G et al. Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy 2014; 10: 2122–2142.
Sasaki K, Tsuno NH, Sunami E, Kawai K, Hongo K, Hiyoshi M et al. Resistance of colon cancer to 5-fluorouracil may be overcome by combination with chloroquine, an in vivo study. Anti-Cancer Drugs 2012; 23: 675–682.
Saleem A, Dvorzhinski D, Santanam U, Mathew R, Bray K, Stein M et al. Effect of dual inhibition of apoptosis and autophagy in prostate cancer. The Prostate 2012; 72: 1374–1381.
Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle 2010; 9: 3515–3533.
Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest 2009; 119: 1109–1123.
Cano CE, Hamidi T, Garcia MN, Grasso D, Loncle C, Garcia S et al. Genetic inactivation of Nupr1 acts as a dominant suppressor event in a two-hit model of pancreatic carcinogenesis. Gut 2014; 63: 984–995.
Garcia MN, Grasso D, Lopez-Millan MB, Hamidi T, Loncle C, Tomasini R et al. IER3 supports KRASG12D-dependent pancreatic cancer development by sustaining ERK1/2 phosphorylation. J Clin Invest 2014; 124: 4709–4722.
Yuan JS, Reed A, Chen F, Stewart CN Jr . Statistical analysis of real-time PCR data. BMC Bioinformatics 2006; 7: 85.
Acknowledgements
This work was supported by La Ligue Contre le Cancer, INCa, Canceropole PACA, DGOS (labellisation SIRIC) and INSERM to JLI, National Institutes of Health grants DK52913 (RU), CA178627 (GL), the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), and the Mayo Foundation (RU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by GM Fimia
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Loncle, C., Molejon, M., Lac, S. et al. The pancreatitis-associated protein VMP1, a key regulator of inducible autophagy, promotes KrasG12D-mediated pancreatic cancer initiation. Cell Death Dis 7, e2295 (2016). https://doi.org/10.1038/cddis.2016.202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2016.202