Abstract
As apoptotic pathways are commonly deregulated in breast cancer, exploring how mammary gland cell death is regulated is critical for understanding human disease. We show that primary mammary epithelial cells from protein kinase C delta (PKCδ) −/− mice have a suppressed response to apoptotic agents in vitro. In the mammary gland in vivo, apoptosis is critical for ductal morphogenesis during puberty and involution following lactation. We have explored mammary gland development in the PKCδ −/− mouse during these two critical windows. Branching morphogenesis was altered in 4- to 6-week-old PKCδ −/− mice as indicated by reduced ductal branching; however, apoptosis and proliferation in the terminal end buds was unaltered. Conversely, activation of caspase-3 during involution was delayed in PKCδ −/− mice, but involution proceeded normally. The thymus also undergoes apoptosis in response to physiological signals. A dramatic suppression of caspase-3 activation was observed in the thymus of PKCδ −/− mice treated with irradiation, but not mice treated with dexamethasone, suggesting that there are both target- and tissue-dependent differences in the execution of apoptotic pathways in vivo. These findings highlight a role for PKCδ in both apoptotic and nonapoptotic processes in the mammary gland and underscore the redundancy of apoptotic pathways in vivo.
Similar content being viewed by others
Main
Apoptosis is an active process of cell death that has a key role in the development and maintenance of tissue homeostasis.1 Mammary gland development occurs primarily in the postnatal period through the process of branching morphogenesis during puberty, and massive proliferation and secretory differentiation during pregnancy.2 During puberty, terminal end buds (TEBs) form at the leading edge of the growing ducts and bifurcate to produce the branched ducts characteristic of the mature virgin. TEBs are composed of a layer of highly proliferative cap cells on their distal surface, surrounding a mass of luminal body cells that undergo high levels of apoptosis resulting in luminal hollowing.3, 4 Multiple B-cell leukemia/lymphoma 2 (Bcl-2) family members are expressed during branching morphogenesis, including antiapoptotic B-cell leukemia/lymphoma X (Bcl-x), Bcl-2 and B-cell leukemia/lymphoma w (Bcl-w), as well as the proapoptotic Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak) proteins.5 Mice in which the Bcl-2 gene is overexpressed have abnormal ductal development, whereas mice in which the proapoptotic protein, Bcl-2-interacting mediator of death (Bim), is deleted show delayed apoptosis in the TEBs, supporting a role for apoptosis during ductal morphogenesis.3, 6
In addition to early luminal hollowing, apoptosis is also important for the clearance of epithelial cells during mammary gland involution. Following the cessation of lactation, large numbers of secretory mammary epithelial cells (MECs) are deleted by apoptosis, returning the gland to its prepregnancy state.7, 8 Quantitative morphometric analysis of epithelial cell apoptosis and apoptotic cell clearance during mammary gland involution suggests that these processes occur rapidly after forced weaning and are largely complete by 72–96 h.8 Gene array studies show an early transient increase in the expression of death receptor ligands and their receptors starting 12 h after weaning, while increased expression of regulators of the intrinsic apoptotic pathway, including Apaf1, Bcl-x, Bak and Bax, and suppression of the death inhibitory proteins, Bcl-2 and Bcl-w, was observed at 24–96 h of involution.5, 9, 10 In mice, loss of Bax or overexpression of Bcl-2 results in suppression of alveolar cell apoptosis,11 whereas deletion of Bcl-x accelerates apoptosis during involution.12
Protein kinase Cδ (PKCδ) is an ubiquitously expressed isoform of the PKC family of serine/threonine kinases.13 Studies have identified diverse roles for this signaling molecule in control of immunity,14, 15 apoptosis,16 and cell migration.17 Furthermore, its reduced expression in some human tumors suggests that it may function as a tumor suppressor.18, 19 Our laboratory and others have shown an essential role for PKCδ in epithelial cell apoptosis induced by genotoxins, other cell toxins, and death receptors.16, 20, 21, 22 The central role PKCδ has in epithelial cell apoptosis suggests that PKCδ may contribute to the regulation of apoptosis in the mammary gland in vivo. In this study, we have explored this hypothesis using mice in which the PKCδ gene has been disrupted (δKO). We show that PKCδ regulates branching morphogenesis through nonapoptotic mechanisms in early mammary gland development. During mammary gland involution, however, the absence of PKCδ results in delayed apoptosis. Our studies also show that apoptosis in the thymus displays a similar differential sensitivity to apoptotic signals, suggesting a redundancy of apoptotic pathways in vivo.
Results
PKCδ expression in the mouse mammary gland
Development and remodeling of the mammary gland during puberty and involution require apoptosis, and thus this tissue is a useful model for exploring regulation of cell death in vivo. As shown in Figure 1, PKCδ protein is expressed at all stages of the mammary gland developmental cycle, with the highest levels seen during mid-pregnancy and during involution. As expected, no PKCδ expression was detected in tissues from δKO mice. In contrast to PKCδ, the expression of PKCα does not vary during the mammary developmental cycle (Figure 1). The mammary gland is composed of epithelial, adipose, and connective tissue. Expression of PKCδ in MECs was verified by immunoblot of primary MECs isolated from postpubertal δWT and δKO mice (data not shown). Differential regulation of PKCδ expression in the mammary gland suggests that it may contribute to the dynamic changes seen in this gland during pregnancy and involution.
Expression of PKCδ in the mammary gland. Mammary glands were harvested from δWT or δKO mice at the time points indicated and PKCδ or PKCα expression was determined by immunoblot analysis as described in Materials and Methods section. Blots were stripped and reprobed for total ERK expression (p44 and P42) as a loading control. Samples are as follows: 6 and 10 week virgin mice (6W, 10W); pregnancy day 5 and 15 (P5, P15); lactation day 2 (L2), and involution days 2, 4 and 6 (I2, I4 and I6). Representative data from three or more mice per time point are shown
Suppression of apoptosis in primary MECs from δKO mice
We have previously shown that the loss of PKCδ protects salivary gland cells in vivo from irradiation-induced cell death; however, the contribution of PKCδ to developmental or physiological programs of cell death is not known.22 To assess whether PKCδ is required for apoptosis in MECs, we investigated the effects of etoposide and integrin detachment on MECs isolated from δKO or δWT mice. Primary MECs were cultured in vitro and treated with etoposide. Activation of caspase-3 in MECs from δKO mice was suppressed by about 50% compared with MECs isolated from δWT mice, similar to what we have previously observed in primary salivary epithelial cells22 (Figure 2a). Loss of integrin engagement (anoikis) induces apoptosis in epithelial cells and may be an important apoptotic mechanism during mammary gland involution. To determine if anoikis is a PKCδ-dependent process, MECs were detached from the culture dish and plated on polyhema-coated plates to prevent reattachment. Caspase-3 activation occurs within 4 h after plating; however, activation of caspase-3 was greatly diminished in δKO MECs as compared with δWT MECs (Figure 2b). Together, this suggests that PKCδ regulates multiple apoptotic pathways relevant to mammary gland development and maintenance.
PKCδ regulates apoptosis in MECs in vitro. MECs were prepared from δWT and δKO mice as described in Materials and Methods section. (a) Primary MECs were treated with etoposide for 16 h, harvested and caspase-3 activity assayed as described in Materials and Methods section; δWT, light gray bars; δKO, dark gray bars; Ut=untreated. Results are the average of triplicate measurements±S.E.M. A representative experiment is shown (n=3); *P<0.02 by Student's two-tailed t-test. (b) Primary δWT or δKO MECs were plated on polyhema-coated dishes to prevent attachment and harvested at the indicated times. Top, immunoblot for active caspase-3; bottom, blots were stripped and immunoblotted for ERK as control for protein loading and transfer. Representative immunoblots are shown (n=3)
Development of the mammary gland during puberty in δKO mice
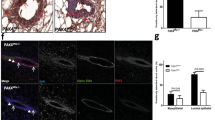
Before the onset of puberty the mouse mammary gland consists of quiescent rudimentary ducts. Under the influence of pubertal hormones TEBs form at the leading edge of the growing ducts and undergo several bifurcation events to produce the ducts characteristic of the mature virgin. Body cells within the TEB undergo apoptosis resulting in a hollow duct for milk flow. To determine if PKCδ contributes to branching morphogenesis, we examined glands from δKO and δWT mice at 4, 5, 6 and 10 weeks of age (Figure 3). As seen in Figure 3A, the morphology of mammary glands from δKO and δWT mice at these developmental time points appears to be similar. Immunohistochemical analysis of proliferation and apoptosis in TEBs from 5-week-old δWT and δKO mice also showed comparable Ki-67 (proliferation) and active caspase-3 (apoptosis) staining (Figure 3B). However, examination of the ductal structure in mammary gland whole mounts revealed a ductal architecture that is more simplistic and open in δKO mammary glands relative to δWT mammary glands (Figure 3C). Notably, ductal branching appeared to occur less frequently in δKO mammary glands compared with δWT glands, as relatively long ducts without side branches were commonly observed in the δKO mammary glands (Figure 3C; arrows). When these effects were quantified we found a small, but significant increase in the distance between primary branch points at 4, 5 and 6 weeks (Figure 3D). As overall duct length did not differ between the δKO and WT mice (data not shown), we conclude that branching occurs less frequently in the mammary glands of δKO mice, indicating that PKCδ contributes to branching morphogenesis in vivo. However, other pathways eventually compensate for the loss of PKCδ, as the defect in branching in the δKO mice was transient, with no observable differences at 10 weeks (data not shown). Importantly, as apoptosis is not reduced, the decreased branching observed in δKO mice during pubertal development indicates an apoptosis-independent function for PKCδ in mammary gland development.
The loss of PKCδ leads to defective mammary gland branching morphogenesis. Immunohistochemistry, whole mounts and analysis of branching frequency were performed as described in Materials and Methods section. (A) Representative pictures ( × 40) of mammary glands from 5, 6 and 10 weeks virgin δWT and δKO mice stained with H&E. (B) Sections ( × 400) through one δWT (a–c) and one δKO (d–f) TEB stained with H&E (a, d), or with antibodies to cleaved caspase-3 (b, e) or Ki-67 (c, f). Inset, digital magnification of area boxed in blue to show cleaved caspase-3-positive cells (b, e) or Ki67-positive cells (c, f). (C) Representative whole mounts of 4th mammary glands from 4-, 5- and 6-week-old δWT and δKO mice stained with carmine alum. Arrows identify areas of decreased branching in δKO mammary glands as compared with δWT. (D) The distance between branch points was quantified from 4-week (n=7), 5-week (n=6) and 6-week (n=5) old δWT (light gray bars) and δKO (dark gray bars) mice. The difference between branching frequency in δWT and δKO is significant over 4 weeks (*P<0.02), 5 weeks (**P<0.03), and 6 weeks (***P<0.03) by Student's two-tail t-test
Mammary gland involution in δKO mice
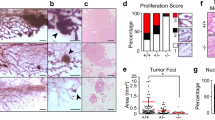
To determine if PKCδ contributes to apoptosis during mammary gland involution, we used a forced-weaning model in which pups were removed from dams 9 days after parturition. In this model, lactation is fully established before pup removal and involution occurs over approximately 2–3 weeks, after which the gland resembles its prepregnant state. Early involution (days 1–4) is associated with suppression of milk protein genes and loss of up to 80% of the secretory epithelial cells.8 Latter stages are characterized by extensive tissue remodeling. Mammary gland tissue was harvested from δWT and δKO mice on days 1 through 8 after weaning (I1 to I8) and apoptotic cells were identified by staining with an antibody that detects active caspase-3. At day 1 of involution (Figure 4a; I1), there was some shedding of apoptotic epithelial cells into the lumen, although this is more evident by involution day 2 (Figure 4a; I2). In mammary glands from δWT mice, the proportion of cells showing caspase-3 activation peaks at involution day 4 and declines thereafter. Meanwhile, mammary glands from δKO mice have peak levels of apoptosis at involution day 6, a 2-day delay compared with δWT. Quantification of active caspase-3-positive cells (Figure 4b) shows that apoptosis is significantly reduced in mammary glands from δKO mice compared with δWT mammary glands at day 2 (36% decrease), day 4 (21% decrease) and day 8 (38% decrease) of involution. In contrast, at involution day 6, activation of caspase-3 in δKO mice is significantly increased relative to δWT mammary glands. These studies suggest that the kinetics of activation of caspase-3 during involution is delayed in the δKO mice relative to δWT mice.
Loss of PKCδ delays activation of caspase-3 during early involution. (a) Mammary gland tissue was harvested from δWT and δKO mice at involution days 1–8 (I1 to I8), sectioned, and stained with an antibody to active-caspase-3 to identify apoptotic cells. Representative pictures ( × 200 magnification) of each time point are shown (n=6 or more mice per time point). (b) Caspase-3-positive cells were quantified as a percent of total epithelial cells per section. A minimum of 1000 cells from five fields were counted per mouse; six or more mice were analyzed per time point. δWT, light gray bars; δKO, dark gray bars; *differs from δWT P<0.05, ***P<0.001, **P<0.03 by Student's two-tailed t-test
To determine if delayed activation of caspase-3 correlates with a delay or suppression of mammary gland involution, we examined mammary gland histology in δWT and δKO mice up to 21 days after weaning. Lactating glands from both genotypes show normal alveolar development and secretory activation (Figure 5, L9). Following pup withdrawal, milk accumulates within the glands (Figure 5, I1 and I2). Although the histology of mammary glands from δWT and δKO mice appears similar at these stages, lipid droplet accumulation in the δKO glands at I1 was consistently greater compared with glands from δWT mice (see inset). By involution day 3, most of the alveolar structures had collapsed (Figure 5, I3). Mid-to-late involution is characterized by remodeling of the gland, which is complete by about 21 days. A comparison of mammary gland histology at involution days 4, 6, 8, 14 and 21 in δWT and δKO mice shows a loss of secretory alveoli, a reduction in ductal structures, and an increased ratio of fat to epithelial cells. Histologically, mammary glands from δWT and δKO mice appear to be very similar, and glands from both genotypes are fully regressed by day 21 (Figure 5, I21). However, it was noted that a subset of mammary glands from δKO mice at involution days 4, 6 and 8 tended to have slightly more epithelial cells and to be more pleiomorphic with regard to adipocyte size and shape compared with glands from δWT mice, suggesting that PKCδ may have a role in the composition or organization of the mammary gland stroma.
Involution in mammary glands from δKO mice. Mammary glands from δWT and δKO mice at involution days 1–21 (I1 to I21) were stained with H&E as described in Materials and Methods section. Representative pictures ( × 100 magnification) are shown from six or more mice analyzed per time point. Inset, × 200 magnification to show lipid accumulation
Signal transducer and activator of transcription 3 (STAT3) is a critical regulator of mammary gland involution, and mice with a conditional deletion of STAT3 in the mammary gland show impairment of epithelial cell apoptosis and delayed involution.23, 24, 25 STAT3 activation, as determined by phosphorylation on Y705, was analyzed to assess if the delay in caspase-3 activation during involution in δKO glands is due to aberrant STAT3 signaling. Similar levels of activated STAT3 are seen in mammary glands from δWT and δKO mice at L2 and I2, and slightly reduced in mammary gland tissue from δKO mice at I4 (Figure 6a). To further address whether STAT3 activation is altered in the mammary glands of δKO mice, we analyzed STAT3 Y705 phosphorylation in primary MECs treated with leukocyte inhibitory factor (LIF) and interleukin-6 (IL-6), cytokines critical for activation of STAT3 in the mammary gland during involution.26 STAT3 activation was nearly identical in δWT and δKO MECs treated with IL-6 or LIF (Figure 6b). Taken together, our studies indicate that loss of PKCδ results in delayed activation of caspase-3 during involution, and that this is not due to a defect in STAT3 activation.
STAT3 activation in mammary glands from δKO mice. (a) Protein lysates were prepared from δWT and δKO mammary glands and expression of phosphorylated STAT3 (pY705), total STAT3 and ERK were analyzed by immunoblot as described in Materials and Methods section. (b) Primary MECs from δWT and δKO mice were starved for 24 h in serum-free DMEM/F12 followed by stimulation with either IL-6 or LIF (50 ng/ml) for the indicated time; U=untreated. Cells were harvested and expression of pY705 STAT3 and total STAT3 was analyzed by immunoblot. Representative blots are shown; each experiment was repeated three or more times
Thymic apoptosis in δKO mice
Although loss of PKCδ results in a delay in caspase-3 activation in the mammary gland, involution proceeds normally in these mice. In contrast, irradiation-induced caspase-3 activation is reduced by >60% in the parotid glands of δKO mice.22 This suggests that in vivo apoptotic pathways induced by agents that damage DNA are highly selective for PKCδ, whereas other inducers of apoptosis such as those that regulate development may be less selective. To further explore the selectivity of apoptotic pathways for PKCδ, we compared caspase-3 activation in the thymus in response to irradiation and dexamethasone treatment. Glucocorticoids have been shown to induce apoptosis and cause acute thymic involution in rodents, and this pathway of involution may be relevant physiologically in response to stress and inflammation.27, 28 δKO or δWT mice were injected with dexamethasone, the thymus was harvested after 2 h, and active caspase-3 was assayed by immunhistochemistry. As seen in Figure 7a, dexamethasone is a potent inducer of apoptosis in the thymus of both δKO or δWT mice; however, no significant difference in caspase-3 activation was found. In contrast, in response to irradiation of the thymus, caspase-3 activation is potently induced in δWT, but not δKO mice (Figure 7b). This finding suggests that irradiation-induced apoptosis is highly dependent on PKCδ, whereas collateral apoptotic pathways may be recruited to regulate cell death in response to other signals, including developmental signals.
PKCδ and apoptosis in the thymus. Thymic involution was induced by dexamethasone (a) or irradiation (b) as described in Materials and Methods section; UT=untreated. Tissues were removed and apoptosis detected by immunhistochemistry for active caspase-3. Shown is the average of active caspase-3-positive cells per × 200 field. Six fields were counted per mouse; n=5 mice per each genotype for dexamethasone and four mice for irradiation. Significantly different from δWT, *P=0.0001; δWT, light gray bars; δKO, dark gray bars
Discussion
PKCδ is required for apoptosis in response to cell injury;21 however, its contribution to apoptosis during development and tissue remodeling has not been addressed. In this study, we have explored a role for PKCδ in the apoptosis of primary MECs during branching morphogenesis and involution. In vitro, apoptosis in primary MECs derived from δKO mice is reduced, and in vivo, activation of caspase-3 during mammary gland involution is delayed in δKO mice. Loss of PKCδ also results in decreased branching during pubertal development of the mammary gland; however, this is likely due to a nonapoptotic function of PKCδ. These phenotypic changes are transient presumably reflecting the redundancy of apoptotic pathways in vivo and the value of proper mammary gland development to species survival.
Branching morphogenesis requires a delicate balance between apoptotic and proliferative signals to effectively establish ductal outgrowth and luminal hollowing. During puberty, mammary glands from δKO mice are similar in organization to δWT glands, with hollow lumens and correct TEB and ductal architecture. Proliferation and caspase-3 activation also appear to be similar in the TEBs of δKO and δWT mammary glands. This suggests that PKCδ either does not have a significant role in the maintenance of cell growth/death within the TEB or that loss of PKCδ is compensated for by other cell death mechanisms. Brugge and co-workers6 have shown that loss of the proapoptotic factor, Bim, suppresses apoptosis within the TEB resulting in transient luminal filling; however, the ducts eventually hollow out by a caspase-independent mechanism and resemble wild-type mammary glands. Similarly, overexpression of the antiapoptotic proteins Bcl2 or Bcl-XL suppresses apoptosis in three-dimensional cultures of MCF10A cells, but lumen formation still occurs.29 As mammary gland development and function is critical to species survival, there is likely to be evolutionary pressure to provide alternative pathways to assure proper mammary gland function. In the context of ductal morphogenesis, these may include alternative regulators of apoptosis, as well as nonapoptotic cell death pathways such as autophagy.6
Whole mount stains of mammary glands from δKO mice during puberty show reduced branching during early ductal morphogenesis; however, by later stages these glands were indistinguishable from their WT counterparts. Altered branching morphogenesis has been observed in several other mouse models within the epithelial and stromal compartments. Although estrogen receptor,30 progesterone receptor,31 matrix metalloproteinase-232 and Src-133 have been shown to be essential for both branching and ductal outgrowth, other factors, such as eotaxin,34 primarily regulate branching. Notably, within the diverse variety of mouse models that exhibit a branching phenotype, there are very few cases in which branching morphogenesis is halted altogether, highlighting the complexity of this process. While changes in proliferation and apoptosis can affect TEB shape and its ability to drive ducts through the fat pad, ductal elongation was not affected in δKO mice. This confirms that alterations in branching were not due to deficiencies in proliferation or cell death within the TEB. More importantly, these findings implicate PKCδ in a nonapoptotic role during ductal morphogenesis.
Our previous studies show that in salivary epithelial cells PKCδ regulates apoptosis upstream of cytochrome c release and caspase activation in response to genotoxins and other cell damaging agents.21, 22 In this study, we show that apoptosis induced by etoposide is similarly suppressed in primary MECs from δKO mice. Interestingly, activation of capsase-3 in anoikis, a process possibly relevant to physiological modes of apoptosis, is also suppressed in δKO MECs. Our current studies show that PKCδ contributes to the activation of caspase-3 during mammary gland involution in vivo; however, suppression of caspase-3 activation is much greater in cultured primary MECs from δKO mice and in irradiated δKO salivary glands in vivo.22 This may be explained in part by the fact that tissue cell death peaks by 24 h postirradiation, while involution occurs over 1–4 days, possibly allowing activation of redundant cell death programs to insure successful completion. Alternatively, several studies indicate that cell death during involution occurs in a series of sequential steps, perhaps mediated by distinct cell death pathways.7, 10 PKCδ predominantly facilitates intrinsic- or mitochondrial-mediated cell death, whereas microarray data suggest that death receptor or extrinsic cell death pathways regulate the initial wave of apoptosis during involution. If intrinsic pathways function as a back-up mechanism of cell death during involution, this may explain the relatively minor changes in involution seen in the δKO mouse.
Nuclear accumulation of PKCδ is an early event in genotoxin-induced apoptosis that results in the activation of caspase-3 and amplification of the apoptotic signal.35, 36 Our current studies suggest that apoptosis in vivo is differentially dependent on PKCδ. This suggests that upstream regulators of apoptosis, such as PKCδ, may be differentially activated in a signal-specific manner. In this regard, we show that dexamethasone induces caspase-3 activation in the thymus to a similar extent in both δKO and δWT mice, while in response to irradiation, caspase-3 activation is induced in δWT and not δKO mice. This supports the role of PKCδ in DNA damaging pathways, whereas alternative apoptotic pathways maybe recruited to facilitate cell death in response to other stimuli.
The loss of PKCδ results in small changes across both mammary gland pubertal growth and involution. This suggests that the role of PKCδ in the mammary gland is not restricted to apoptosis, as a reduction in apoptosis is not likely to explain the decreased ductal branching or decreased lactation we observe. Furthermore, subtle differences in lipid composition are evident in the mammary glands of δKO mice, suggesting that PKCδ may have additional roles in mammary gland homeostasis. This suggests that mammary gland development and involution are complex biological processes that are essential for the maintenance and propagation of mammalian species, thus apoptosis is likely to be regulated at multiple levels to assure its proper execution.
Materials and Methods
Animals
The PKCδ −/− mouse (δKO) on the C57/Bl6 background has been previously described.15 Animals were maintained at the University of Colorado Denver at Anschutz Medical Campus in accordance with Laboratory Animal Care guidelines and protocols. These studies were conducted with approval of the University of Colorado Denver Institutional Animal Use and Care Committee. Wild-type littermates (δWT) were used for all studies. For analysis of mammary gland involution, pups were removed from mothers at day 9 of lactation.
Mammary gland preparation
Mammary glands were harvested from δWT or δKO mice and processed for whole mount analysis or immunohistochemistry. For analysis of mammary gland whole-mounts, mammary glands (#4) were spread on microscope slides and fixed overnight in 10% formalin. Tissues were hydrated, stained with carmine alum overnight, dehydrated and cleared in xylene for 2 h. Slides were analyzed on a dissecting scope and digital pictures were acquired using SPOT imaging software. For quantification of branching frequency, the total duct length of every primary branch, from nipple to distal TEB, was measured in microns and divided by the total number of branch points within the gland.
For histological and immunohistochemical analysis, contralateral #4 glands were fixed in 10% formalin, dehydrated, and embedded in paraffin. Five μm sections were stained with hematoxylin and eosin (H&E). Immunohistochemistry for active caspase-3 and Ki67 were performed as previously described using primary antibodies purchased from Cell Signaling Technology, Beverly, MA, USA.22 Digital images were acquired using SPOT imaging software.
Immunoblot analysis
Immunoblots were preformed as previously described.20 Super Signal West Pico Luminol/Enhancer Solution (Pierce, Rockford, IL, USA) was used for detection of the signal. Sources of antibodies were as follows: PKCδ and PKCα (Santa Cruz Biotechnology, Santa Cruz, CA, USA), STAT3, pY705STAT3, active caspase-3, Ki-67 and extracellular signal-regulated kinase (Cell Signaling Technology).
Primary cell culture
Mammary glands removed from δWT or δKO mice (8–14 weeks) were minced and digested in Dulbecco's modified Eagle medium/F12 (DMEM/F12) containing 2 mg/ml collagenase-B, 100 U/ml Hyaluronidase, 100 U/ml penicillin-streptomycin (pen/strep), and 100 U/ml Gentamicin at 37° for 3 h. Primary MECs were isolated by a series of spins at 1500 and 800 r.p.m. and then plated on collagen 1 (Sigma-Aldrich, St. Louis, MO, USA)-coated plates (2.5 × 105/cm2) in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 1 mg/ml fetuin (Sigma-Aldrich), 2.5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 2.5 μg/ml epidermal growth factor (EGF), 25 μg/ml gentamicin and 50 U/ml Pen/Strep. After 48 h, cells were transferred to growth media (DMEM/F12 supplemented with 2.55 μg/ml insulin, 0.5 μg/ml hydrocortisone, 2.5 μg/ml EGF, 25 μg/ml gentamicin, 50 U/ml pen/strep and 10% FBS). For induction of anoikis, cells were trypsinized and plated on dishes coated with Polyhema (Sigma-Aldrich). Before treatment with LIF or IL-6, MECs were plated on Matrigel (BD Biosciences, Bedford, MA, USA) for 24 h in DMEM/F12 supplemented with 10% fetal calf serum, 1 mg/ml fetuin (Sigma-Aldrich), 2.5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 2.5 μg/ml EGF, 25 μg/ml gentamicin and 50 U/ml pen/strep, followed by incubation in serum-free growth media for an additional 24 h. LIF was purchased from Calbiochem (San Diego, CA, USA) and IL-6 was purchased from Chemicon (Billerica, MA, USA). DMEM/F12 and FBS were purchased from HyClone (Logan, UT, USA); all other reagents for tissue culture were from Invitrogen (Carlsbad, CA, USA) unless noted otherwise.
Caspase-3 activity
Caspase-3 activity was quantified using a Biomol Quantizyme Colormetric Assay Kit (BioMol, Plymouth Meeting, PA, USA) as previously described.22 Briefly, caspase-3 activity in 30 μg of cell lysate was measured by cleavage of Ac-DEVD-pNA colormetric substrate, and absorbance at A405 was quantified in a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA) at 10 min intervals for 7 h.
Apoptosis in the thymus
For dexamethasome-induced involution, 6-week-old δWT or δKO male mice (n=5) were intraperitoneally injected with 0.2 mg dexamethasone (Sigma-Aldrich) dissolved in 100 μl endotoxin-free phosphate-buffered saline (PBS) or 100 μl endotoxin-free PBS alone. Two hours after injection, mice were killed and the thymus glands harvested. For irradiation-induced involution, 6-week-old δWT or δKO male mice (n=4) were subjected to 2Gy irradiation using a cobalt source. Twenty-hours after irradiation, mice were killed and the thymus glands harvested and processed for histology and immunohistochemistry as described.
Miscellaneous
Etoposide was purchased from Sigma-Aldrich and dissolved in dimethylsulfoxide.
Conflict of interest
Drs. Anderson, Neville and Reyland's work has been funded by the NIH. Dr. Nakayama, Ms. Allen-Petersen and Ms. Miller declare no potential conflict of interest.
Abbreviations
- PKCδ:
-
protein kinase C delta
- Bcl-2:
-
multiple B-cell leukemia/lymphoma 2
- TEBs:
-
terminal end buds
- Bcl-x:
-
B-cell leukemia/lymphoma X
- Bcl-w:
-
Bcl-2 and B-cell leukemia/lymphoma w
- Bax:
-
proapoptotic Bcl-2-associated X protein
- δKO:
-
PKCδ gene has been disrupted
References
Richert MM, Schwertfeger KL, Ryder JW, Anderson SM . An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 2000; 5: 227–241.
Silberstein GB . Postnatal mammary gland morphogenesis. Microsc Res Tech 2001; 52: 155–162.
Humphreys RC . Programmed cell death in the terminal endbud. J Mammary Gland Biol Neoplasia 1999; 4: 213–220.
Sternlicht MD . Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 2006; 8: 201.
Metcalfe AD, Gilmore A, Klinowska T, Oliver J, Valentijn AJ, Brown R et al. Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J Cell Sci 1999; 112 (Part 11): 1771–1783.
Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS . BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell 2007; 12: 221–234.
Stein T, Salomonis N, Gusterson BA . Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia 2007; 12: 25–35.
Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM . Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod 2008; 78: 586–594.
Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res 2004; 6: R75–R91.
Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ . Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res 2004; 6: R92–R109.
Schorr K, Li M, Bar-Peled U, Lewis A, Heredia A, Lewis B et al. Gain of Bcl-2 is more potent than bax loss in regulating mammary epithelial cell survival in vivo. Cancer Res 1999; 59: 2541–2545.
Walton KD, Wagner KU, Rucker III EB, Shillingford JM, Miyoshi K, Hennighausen L . Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech Dev 2001; 109: 281–293.
Newton AC . Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 2001; 101: 2353–2364.
Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A . Regulation of B-cell survival by BAFF-dependent PKCdelta-mediated nuclear signalling. Nature 2004; 431: 456–461.
Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature 2002; 416: 865–869.
Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G et al. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest 2001; 108: 1505–1512.
Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI et al. Suppression of cell migration by protein kinase Cdelta. Oncogene 2005; 24: 3067–3072.
D’Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF . The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene 2006; 25: 378–386.
Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP . Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol 2008; 39: 21–29.
Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO . Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem 1999; 274: 19115–19123.
Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME . PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem 2001; 276: 29719–29728.
Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME . Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem 2006; 281: 9728–9737.
Zhao L, Hart S, Cheng J, Melenhorst JJ, Bierie B, Ernst M et al. Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. J Biol Chem 2004; 279: 44093–44100.
Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev 1999; 13: 2604–2616.
Humphreys RC, Bierie B, Zhao L, Raz R, Levy D, Hennighausen L . Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology 2002; 143: 3641–3650.
Watson CJ . Stat transcription factors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 2001; 6: 115–127.
Ahmed SA, Sriranganathan N . Differential effects of dexamethasone on the thymus and spleen: alterations in programmed cell death, lymphocyte subsets and activation of T cells. Immunopharmacology 1994; 28: 55–66.
Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML . Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol 1995; 130: 157–167.
Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS . The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 2002; 111: 29–40.
Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 1996; 51: 159–186.
Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA . A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA 1998; 95: 5076–5081.
Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol 2003; 162: 1123–1133.
Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW . Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 1998; 279: 1922–1925.
Gouon-Evans V, Rothenberg ME, Pollard JW . Postnatal mammary gland development requires macrophages and eosinophils. Development 2000; 127: 2269–2282.
DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME . Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J Biol Chem 2007; 282: 22307–22314.
Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME . Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene 2008; 27: 3045–3053.
Acknowledgements
We appreciate the technical assistance of Dr. Harriet Watkin, Rachelle Kalkofen, Valerie Burns, and Andrew Lewis, and the intellectual contributions of Drs. Pepper Schedin, Peter Henson and James McManaman. These studies were supported by grant PO1-HD38129.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Rights and permissions
This article is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Allen-Petersen, B., Miller, M., Neville, M. et al. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis 1, e17 (2010). https://doi.org/10.1038/cddis.2009.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2009.20
Keywords
This article is cited by
-
Fur removal promotes an earlier expression of involution-related genes in mammary gland of lactating mice
Journal of Comparative Physiology B (2023)
-
Co-dependency of PKCδ and K-Ras: inverse association with cytotoxic drug sensitivity in KRAS mutant lung cancer
Oncogene (2017)
-
Protein kinase Cδ is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer
Oncogene (2014)
-
Analysis of apoptosis methods recently used in Cancer Research and Cell Death & Disease publications
Cell Death & Disease (2012)
-
Cell death in disease: from 2010 onwards
Cell Death & Disease (2011)