Abstract
There is evidence that naturally occurring antibodies directed against Aβ (nAbs-Aβ) have a role in Aβ-metabolism and Aβ-clearance. The presence of nAbs-Aβ leads to a reduction in amyloid fibrillation and thus a reduction in their toxicity. We investigated the effects of nAbs-Aβ in respect to oligomerization and used the Tg2576 transgenic mouse model in order to investigate the rapid effect with a single-dose (24 h) on oligomer breakdown and cytokine secretion along with immunohistochemical characterization of synaptic plasticity. nAbs-Aβ were able to reduce toxic oligomer concentration with an increase in Aβ-monomers. Cytokine secretion was significantly reduced. Synaptic plasticity was also improved after administration of nAbs. Finally, single treatment lead to a significant improvement in cognition. This study demonstrates the efficacy of nAbs-Aβ and presents evidence that several hallmarks of the disease are targeted by nAbs-Aβ.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder and affects ∼20–30 million people worldwide. The histopathological hallmarks of AD are the occurrence of extracellular neuritic and non-neuritic senile amyloid plaques and intracellular neurofibrillary tangles, which are thought to trigger synaptic dysfunction, reduce synaptic density and eventually lead to neuronal cell death.1, 2 Recent evidence points to a crucial role of toxic Aβ oligomers rather than plaque formation in the development of this devastating disorder.3, 4
Schenk and co-workers demonstrated that active immunization directed against β-amyloid (Aβ) reduced the plaque load considerably in an APP transgenic mouse model.5 This groundbreaking discovery led to the development of a variety of approaches towards active immunization with Aβ as well as passive immunization. Passive immunization with murine monoclonal and, to a lesser extent, murine polyclonal Aβ-antibodies in APP transgenic mice, resulted in both reduction of Aβ burden and improvement of cognitive functions.6, 7 Previous studies from our group and from other investigators have recently detected antibodies against Aβ in the serum and consequently in human intravenous immunoglobulin preparations (IVIg); these naturally occurring autoantibodies (nAbs-Aβ) have been shown to inhibit Aβ toxicity and inhibit Aβ oligomerization in vitro.8, 9, 10 Human IVIg are already being tested in clinical phase II/III trials, making them promising therapeutics for AD.11, 12 Further evaluation of their role in AD as well as their physiological importance needs to be clarified.8, 13 Recently, we were able to provide first evidence for a protective function of nAbs-Aβ on oligomer Aβ peptide toxicity and cognition in an animal model for AD after treatment for a 4-week period.14 The aim of the present study is to further elucidate the mechanism of action of affinity-purified nAbs-Aβ 24 h after treatment in vitro and in vivo in an APP transgenic mouse model for AD.
Materials and methods
Purification of nAbs-Aβ
For a detailed description see previous publications.14, 15 Briefly, we used purified human intravenous IgG (Octagam 5%, which was kindly provided by Octapharma AG, Lachen, CH, Switzerland) for the isolation of nAbs-Aβ. For the following isolation steps this preparation was mixed with an equal volume of phosphate-buffered saline (PBS) and loaded directly onto an affinity column. As the Aβ1-40 sequence contains internal lysine residues, which might lead to side reactions in immobilization procedures using amino groups, a specific affinity column was prepared using a cysteine residue attached to the Aβ-N-terminus to ensure homogeneous orientation of peptide molecules on the column support by immobilization through cysteinyl-S-thioether linkage. The azlactone-activated support contains an iodoacetyl group (Ultralink; Perbio, Bonn, Germany) at the end of a hexadecyl-spacer group, which reacted with the cysteinyl-sulfhydryl group to yield a stable thioether linkage in order to reduce steric hindrance and provide maximum binding capacity of the antibodies. For covalent attachment of the Cys-Aβ1-40, 3.7 mg of peptide was dissolved in 50 mM Tris and 5 mM EDTA-Na coupling buffer (pH 8.5) to a final concentration of 0.37 mg ml−1. The solution was added to 1 ml of drained ultralink-iodoacetyl gel and the coupling reaction was performed for 1 h at room temperature under gentle mixing, followed by 30 min reaction time without mixing. An aliquot of 0.5 ml of the Cys-Aβ1-40 coupled support was packed into a column (2.5 ml, MoBiTec, Goettingen, Germany) allowing the solution to drain. The column was washed with 3 ml of coupling buffer, and non-specific binding sites on the gel were blocked for 2 × 45 min with 1 ml of 50 mM L-Cysteine-HCl in coupling buffer. Subsequently the column was washed with 5 ml of 1 M NaCI and 5 ml of 0.l M Na-phosphate, 0.15 M NaCI (pH 7.2) and stored at 4 °C. The gel support (0.5 ml) was transferred into a 15 ml falcon vial using 5 ml PBS and mixed with 5 ml IVIg. After gentle shaking overnight at 4 °C, this suspension was transferred to the column using the effluent to completely rinse the matrix back into the column. The column was washed eight times with 10 ml of PBS followed by two wash cycles with 10 ml ultrapure water.
The bound antibodies were eluted from the column with 10 × 0.5 ml 0.1 M glycine buffer, pH 2.8. Each fraction was collected in a microreaction tube containing 35 μl 1 M Tris-HCl, pH 9. The flow-through of this isolation procedure, which contains IgG depleted of nAbs-Aβ, was also collected and was used as a control in the respective experimental settings.
To maintain the integrity of the antibodies, a neutral pH was adjusted immediately after elution by adding the appropriate amount of Tris-HCI or glycine buffer. To regenerate the column for further use, the column was washed once with 10 ml 10 mM Na-phosphate buffer pH 6.8, followed by two wash cycles with 10 ml of PBS containing 1 M NaCl and finally two wash cycles with 10 ml PBS.
Antibody concentrations in the elution fractions were determined by the Micro BCA Protein Assay Kit method (Pierce; Perbio, Bonn, Germany). The stock solution of 2 mg ml−1 of bovine albumin supplied within the Micro BCATM Kit was used to prepare fresh standard dilutions within the range 40–0.5 μg ml−1. The antibodies were eluted between fractions 1−6, with the highest concentrations in fractions 1 and 3. For quantification of each set of ten elutions, fresh albumin standard dilutions were prepared. Results were read at 562 nm with an ELISA reader. Detailed affinity studies on these isolated IgG have been performed in a recent communication.14
Antibodies and chemicals
The following antibodies were used: antibodies against Aβ (clone 6E10, Gentaur, Brussels, Belgium); species-specific horseradish peroxidase-conjugated secondary antibodies (Cell signaling, Danvers, MA, USA); All remaining chemical compounds were obtained from Sigma-Aldrich (Hamburg, Germany) and Carl Roth (Karlsruhe, Germany) if not otherwise stated.
Aβ oligomerization
Toxic oligomers were synthesized according to a modified protocol by Kayed et al.16 Briefly, 300 μg Aβ1-40/Aβ1-42 were dissolved in 90 μl hexafluoroisopropanol and 210 μl deionized water and diluted with 900 μl 100 mM NaCl, 50 mM Tris, pH 7.4 at 25 °C. The solution was stirred for 48 h at 37 °C. The tubes were weighed and the evaporated alcohol was replaced with 100 mM NaCl, 50 mM Tris, pH 7.4. The Aβ concentration of this oligomer-enriched solution is 56 μM. To study whether nAbs-Aβ interfere with Aβ oligomers, 56 μM oligomer-enriched Aβ was incubated in the presence of 0.3 μM nAbs or solvent (Tris-buffered saline) for 24 h at 37 °C. The effect of nAbs-Aβ on Aβ oligomerization and fibrillization was studied by adding 2 μM nAbs-Aβ or solvent (tris-buffered saline) to 56 μM of solubilized Aβ before oligomerization.
Western blot
Three hundred nanograms Aβ (PSL GmbH, Germany) were loaded on precasted NuPage novex 4–12% Bis-Tris gels (Novex-system, Invitrogen, Darmstadt, Germany). Proteins were transferred onto nitrocellulose membranes (Whatman, Dassel, Germany) using XCell II blot (Invitrogen). Membranes were blocked overnight at 4 °C with Roti Block (Carl Roth) processed according to standardized procedures.
To distinguish between different Aβ isoforms, we used a protocol by Klafki et al.17 Aβ standard samples for Aβ1-38/Aβ1-40/Aβ1-42 were purchased from Bachem, Switzerland. 100 μg of mice brain lysate were loaded per lane.
Soluble APPα and Aβ detection in cerebrospinal fluid (CSF)
Soluble APPα (sAPPα) and Aβ1-x concentrations in CSF were determined by using sAPPα and Aβ1-x ELISA-kits (IBL, Hamburg, Germany).
Cytokine and Aβ measurement in brain lysates and serum samples
Mice brains were lysed in T-PER lysis buffer (Pierce, Rockfort, IL, USA) containing protease inhibitors (Roche Diagnostics, Mannheim, Germany). Cytokine concentrations of brain lysates and serum samples were measured with specific ELISA-kits (R&D, Wiesbaden, Germany) according to the manufacturer’s instructions. Measurements were performed for IL-1β, IL-6, TNF-α and INF-γ. Aβ concentrations were analyzed by Aβ 1-x ELISA (IBL, Hamburg, Germany).
Animals
Twenty to 22-month-old as well as 27 to 30-month-old heterozygous adult female Tg2576 mice expressing mutant APPSWE (695(K670N,M671L)18 under the control of the hamster prion promoter on a hybrid C57Bl/6xSJL background were used. Age- and gender-matched non transgenic wild-type mice (WT) were used as control. Tg2576 and control mice were randomly assigned to groups of 5–6, independent of genotype and treatment on a 12 h light-dark schedule (lights on 07:00–19:00). They had free access to tap water, were fed ad libitum and kept under standard conditions. Tg2576 mice weighed between 30–40 g at the beginning of the experiments. Behavioral experiments were conducted during the light period between 12:00–18:00 h. Sample sizes of the groups were as follows: transgenic (Tg) control n=11, Tg nAbs-Aβ n=12, WT n=11 for 20 to 22-month-old mice. Tg control n=9, Tg nAbs-Aβ n=12 and WT n=6 for 27 to 30-month-old mice.
All animal procedures were approved by the office of the federal state authority of Hessen and the Institutional Animal Care and Use Committee of the University of Marburg.
Administration of nAbs-Aβ to mice
Mice were treated intraperitoneally with nAbs-Aβ (400 μg dissolved in 0.2 ml of PBS) or vehicle (0.2 ml PBS) 24 h before assessing cognition or killing for brain tissue and CSF sampling. To ensure that the treatment period is 24 h, two different groups of animals were treated for cognition experiments and tissue sampling, respectively. Transgenic animals treated with PBS are displayed as Tg Control, nAbs-treated mice as Tg+nAbs-Aβ. Wildtype mice are referred to as WT.
Visuospatial learning task
The object location test (OLT) is based on the spontaneous tendency of rodents to explore an object moved to a new location more often than an object located at the familiar position.19 In the OLT, animals are introduced to two identical objects in an experimental apparatus and, after a delay, are exposed again to the same two objects, while one of them has been displaced to a new location. Animals that remember the previous exposure spontaneously spend more time exploring the object in the new position.
For analysis of OLT, the amount of time spent investigating the object located in the familiar position in the training trial and on the object which had been displaced to a new location in the test trial was recorded for each mouse. In addition, a discrimination ratio (time spent on the object in the novel location vs the total time spent exploring both objects in the test trial) was calculated for each mouse. OLT was reflected by more time interacting with the object in the novel location than with the object in the familiar position and a discrimination ratio above 0.5. Analysis of discrimination ratios was less subject to the influence of individual variability in contact times with objects and provided a measure of the extent of the discrimination between the novel and sample objects. Mice with a total investigation time less than 2 s were excluded from OLT due to reduced exploratory behavior.
Brain tissue and CSF sampling
Twenty-four hours after the injection, animals were anesthetized and CSF was obtained by puncture of the cisterna magna as previously described.20 CSF was immediately frozen at −80 °C. After surgical isolation of mice brains, the cerebellum was removed and the remaining hemispheres bisected. One hemisphere was fixed in 4% neutral buffered formalin for histological analysis, whereas the remaining hemisphere was frozen −80 °C for subsequent analysis.
Synapse detection using the Golgi-Hortega stain
The mouse brain samples were fixed and stained using the rapid Golgi-impregnation method as previously described.21 Briefly, after fixation in 4% neutral buffered formalin, brains were immersed in darkness for 5 days in 3% potassium bichromate, with fresh solution every day. Excess potassium bichromate was whipped away when brain samples were transferred into 2% silver-nitrate solution for 3 days in darkness, with fresh solution every day. Samples were cut in 80 μm thick slices using a vibratome T1000 and mounted with gelatin. Pictures were taken immediately using a TE2000i Microscope from Nikon (Tokyo, Japan). Synaptic spines and axon length were detected manually using the NIS elements BR software (Kingston, UK). Data were exported to Excel (Microsoft, Redmond, WA, USA) for further statistical analysis.
Statistical analysis
All analyses were performed using the statistical software packages SigmaStat (Statcon, Witzenhausen, Germany) or SPSS15.0 for Windows (SPSS GmbH Software, Germany). All results are presented as the mean±s.e. of the mean(s.e.m.).
The effect of acute immunization with nAbs-Aβ on OLT were analyzed using either one-way or two-way analysis of variance, followed by post-hoc Tukey’s t-test for pair wise comparisons. Within-group t-test analysis was performed for each treatment group to show deficits in the object location memory. For all statistical comparisons, the following definitions were used: P<0.05(*), P<0.01(**) or P<0.001(***).
Results
Treatment with nAbs-Aβ increase soluble Aβ monomers
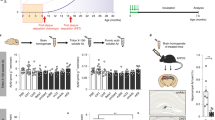
Treatment of 27 to 30-month-old Tg2576 mice with 400 μg nAbs-Aβ for 24 h had no influence on total Aβ1-x levels in brain lysates as well as in the CSF (Figures 1a and b). CSF sAPPα concentration was also not affected in nAbs-Aβ treated compared with control animals (Figure 1c). Interestingly, a considerable increase in soluble intracerebral Aβ monomers could be observed in treated animals compared with control animals, which were only treated with PBS (Figure 1d). Untreated transgenic animals only contained few soluble Aβ monomers in the brain, whereas a 24 h treatment with nAbs-Aβ lead to an increase in the fractions of Aβ1-38, Aβ1-40 and Aβ1-42 (Figure 1e). Densitometric analysis revealed that treatment with nAbs-Aβ increased brain Aβ1-40, Aβ1-42 monomers by a factor of 7.2 (± 2.4, P<0.05) and sevenfold (± 2.3, P<0.05), respectively.
nAbs-Aβ lead to oligomer breakdown in vivo in 27 to 30-month-old Tg2576 mice. Total Aβ concentrations in brain lysates (a) and CSF (b) of treated and control mice are constant. Animals were treated with 400 μg nAbs or vehicle (PBS) for a duration of 24 h. Measurements were performed using ELISA kits. (c) Soluble sAPPα is also unchanged in animals treated with nAbs-Aβ compared both to vehicle treated and control animals. (d) Brain lysates were analyzed using western blot (anti Aβ clone 6E10 monoclonal antibody). Single treatment of transgenic animals with nAbs-Aβ leads to an increase in monomeric Aβ compared with control animals. (e) Further characterization of these monomers demonstrated that Aβ1-40 is most abundant, with Aβ1-42 being less abundant, whereas hardly any Aβ1-38 can be detected (see A). Densitometric analysis for Aβ1-40 (B) and Aβ1-42 (C) showed a sevenfold increase in the treatment group, respectively (anti-Aβ clone 6E10 monoclonal antibody was used to detect Aβ).
In vitro inhibition of Aβ oligomerization by nAbs-Aβ
We performed in vitro experiments in order to further investigate the effect of nAbs-Aβ towards Aβ oligomers and evaluated the effect of nAbs-Aβ on de novo formation of Aβ oligomers using a western blot. Aβ was pretreated before oligomerization with 2 μM nAbs-Aβ. As can be seen in Figure 2a (A), there is a reduction in higher order oligomer formation following the treatment with nAbs-Aβ. Simultaneously, the amount of monomers, dimers and trimers increased following treatment with nAbs-Aβ. Densitometric analysis of the bands is shown in Figure 2a (B). The reduction in fibrils and higher order oligomers is statistically significant, as well as the observed increase in monomers and small oligomers such as dimers and trimers in the treatment group. Next, we analyzed the ability of nAbs-Aβ to break down oligomeric Aβ. Incubation of Aβ oligomers with nAbs-Aβ for 24 h showed no effect compared with solvent only (Figure 2b). Although, further treatment of Aβ oligomers for 24 h with either nAbs-Aβ or solvent showed a pronounced generation of oligomeric Aβ with a molecule size between 30 and 100 kDa due to a longer oligomerization process in comparison to the previous experiment.
In vitro breakdown of Aβ oligomers. Further analysis was performed using western blot (a). Synthetic Aβ1-42 oligomers were synthesized as described and 300 ng were loaded on the gel (A) Densitometric analysis of the bands (B) demonstrated a significant reduction in fibrils and oligomers with a concomitant increase in monomers in the treatment group compared with placebo. Incubation of oligomerized Aβ with nAbs-Aβ or solvent after the oligomerization process shows no effect. Shown are different concentrations of Aβ (2.5–10 μM) which are incubated with 0.3 μM nAbs-Aβ for 24 h. (b) Preincubation of Aβ with nAbs-Aβ before the oligomerization process reduces the generation of multimeric Aβ forms. As a result of this effect monomers and smaller oligomers are increased when Aβ is pretreated with nAbs-Aβ. Controls were incubated with PBS only (in both blots anti Aβ clone 6E10 monoclonal antibody was used to detect Aβ).
Influence of nAbs-Aβ on proinflammatory cytokines in transgenic animals
As there was a reduction in oligomers in vitro following treatment with nAbs-Aβ and an improvement in cognition in the animal model after a single treatment, we evaluated proinflammatory cytokine concentrations in the brain homogenates of WT animals as well as 27 to 30-month-old Tg2576 mice treated with nAbs-Aβ. For all analyzed cytokines we were able to detect an upregulation in transgenic animals (Figure 3). IFN-γ was elevated by a factor of 3.6 compared with WT animals (Figure 3a)(6.10±0.80 vs 21.54±2.07, P<0.001), IL-1β levels (Figure 3b) in Tg2576 mice brain were increased 5.7-fold (2.97±0.27 vs 17.28±1.81, P<0.001), IL-6 (Figure 3c) was increased 2.6-fold (6.55±0.19 vs 17.30±1.77, P<0.001) and TNF-α (Figure 3d) showed a 2.4-fold increase (28.32±0.57 vs 67.95±6.90, P<0.001), respectively. Treatment of Tg2576 mice with nAbs-Aβ led to a significant reduction in the brain cytokine levels. IL-1β concentrations (Figure 3b) were reduced by 63% (± 6%, P<0.001), IL-6 concentration (Figure 3c) was lowered by 64% (± 7%, P<0.001), TNF-α levels (Figure 3d) were reduced by 65% (± 3%, P<0.001) and IFN-γ levels (Figure 3a) were reduced by 60% (± 3%, P<0.001).
Cytokine changes in response to nAbs treatment in 27 to 30-month-old Tg2576 mice. Cytokines were measured using standard ELISA-assays. Measurements were performed for IFN-γ (a), IL-1β (b), IL-6 (c) and TNF-α (d) in brain lysates. For all four cytokines, there is a highly significant increase when comparing WT and untreated animals. Treatment with 400 μg nAbs-Aβ for 24 h leads to a reduction to background secretion in WT animals.
Treatment with nAbs-Aβ lead to restoration of synaptic densities in 22-month-old Tg2576 mice. Synapses were determined as described in the Materials and Methods section. Synapse numbers in relation to the axon number and the proportion of synapses per 100 μm axon length is provided. A significant increase in synapses is readily visible in Tg2576 mice following treatment with nAbs-Aβ in contrast to PBS.
Improvement of visuospatial learning in Tg2576 mice after single administration of nAbs-Aβ
Figure 5a shows the mean interaction time of the mice with the objects during the training trial. No significant differences were found between groups regarding object exploration, indicating intact exploratory behavior. A significant result was found concerning main effect of object (F(1,72)=15.13; P<0.001) and interaction between object and treatment (F(2,72)=4.22; P<0.05) during the test trial. nAbs-Aβ treated 22-month-old Tg2576 mice (P<0.001) and WT control mice (P<0.01) interacted significantly longer with the object in the novel location compared with PBS treated animals (Figure 5b). A discrimination ratio above 0.5 reflects object location memory and between-group analysis revealed that 22-month-old Tg2576 mice immunized with nAbs-Aβ (P<0.001) and WT mice (P<0.001) showed similar relative preferences for the object located to a novel position compared with PBS-treated Tg2576 mice (Figure 5c). Single immunization with nAbs-Aβ significantly ameliorated impairments in visuospatial learning in Tg2576 mice.
Single immunization with nAbs-Aβ leads to rapid improvement of visuospatial learning and memory in 22-month-old Tg2576 mice after 24 h. Object location memory in 20 to 22-month-old Tg2576 nAbs-Aβ treated (n=12), Tg2576 Control (n=11) and WT mice (n=11). (a) No significant differences were found between groups regarding object exploration, indicating intact exploratory behavior during the training trial. (b) Tg2576 nAbs-Aβ treated and WT, but not Tg2576 Control mice, showed a significantly increased interaction time with the object moved to a novel position compared with the object in the familiar location (**P<0.01, ***P<0.001) during the test trial. (c) A discrimination rate above 0.5 reflected preference for the object in the novel position, and Tg2576 nAbs-Aβ treated and WT, but not Tg2576 Control mice, showed a similar preference towards the relocated object (***P<0.001). A within-group t-test analysis revealed an object location memory deficit in Tg2576 control mice. Tg2576 nAbs-Aβ treated mice showed a significant improvement of visuospatial learning and memory after 24 h using the object location memory test. Data presented for all groups are means±s.e.m.
nAbs-Aβ treatment influences synaptic plasticity
From the data shown above we hypothesized that nAbs-Aβ may also improve synaptic plasticity, which is affected by cytokines as well as toxic Aβ oligomers.
To determine the influence of nAbs-Aβ onto synaptic plasticity (formation and degradation of dendritic spines), parts of the brains were impregnated with silver according to the protocol established by Golgi, Cajal and Hortega. Spiny neurons in the visual cortex were identified, and after dendrites of the second level had been pegged, axon length and synaptic spines were determined. Twenty-two-month-old Tg2576 animals treated with nAbs-Aβ displayed significantly higher mean synapse numbers than those treated with PBS. The relation of synapses per axon was significantly higher in the Tg2576 animals treated with nAbs-Aβ comparedwitho the PBS-treated group (Figure 4). A similar significant result was obtained calculating the mean synapse number in relation to 100 μm axon length (Figure 4).
Discussion
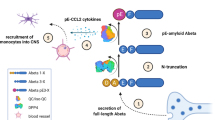
This study investigated the biochemical changes and behavioral effects of a single passive immunization with human nAbs-Aβ 24 h before cognitive testing in a transgenic mouse model for AD. There is increasing evidence for a major role of toxic oligomers in the development of AD.22 According to a study by McLaurin et al.,23 monoclonal antibodies against Aβ were able to interfere with fibrillization and oligomerization processes of Aβ. We therefore investigated the role of nAbs-Aβ in a transgenic animal model of AD, where we were able to show that total brain and CSF Aβ1-x concentration was unaffected by the treatment with nAbs-Aβ. Subsequent western blotting showed an increase in monomeric Aβ following administration of nAbs-Aβ. There are several possible explanations for this observation. It may be possible that nAbs-Aβ in general lead to an increased production of Aβ. The main argument against this hypothesis is the observation that total brain as well as CSF Aβ concentrations were constant. In addition, Yamada et al.24 were able to show that secretase activity was not influenced by antibody treatment with the monoclonal antibody m266. Another possibility is that nAbs-Aβ are able to break oligomers or to dissolve Aβ out of senile plaques. Previous data as well as our in vitro data argue against this possibility.14 Recently, we investigated the application of nAbs-Aβ in a transgenic animal model. In this study, no clearance of amyloid plaques could be observed. In addition, these data point towards a role of nAbs-Aβ to interfere with Aβ peptide toxicity and their ability to oligomerize. Therefore, oligomerization and fibrillization may be inhibited by nAbs-Aβ. In our study we demonstrated that nAbs-Aβ are unable to break down oligomeric as well as fibrillar Aβ in vitro. However, de novo oligomerization to higher aggregated Aβ (n>3) and fibrillization of Aβ could be inhibited by coincubation with nAbs-Aβ. However, we could also observe significant increase in small Aβ oligomers as dimers and trimers following incubation with nAbs-Aβ. Evidence has accumulated that these forms are the toxic aggregation form of Aβ.25, 26 Though, Lesne and others could show, that higher aggregated Aβ, such as oligomers of a size of 56 kDa, are the toxic species that impair memory in the Tg2576 mice model.27 Sherman et al.28 could also detect these Aβ*56 oligomers in brain tissues. Therefore, so far it can be concluded that there is ongoing discussion about which aggregation forms in particular are causative for memory impairment in mouse models of Alzheimers disease as well as in humans. A similar mode of action of antibody treatment in transgenic mice was suggested by Yamada et al.24 At the same time, total brain Aβ levels remained constant. The authors concluded that anti-Aβ antibodies are able to bind soluble Aβ in the brain and thus preventing the accumulation of toxic multimeric forms. This is in line with our observation. Our data also point to a binding of nAbs-Aβ to monomers and small oligomeric forms and a subsequent sequestration, which precludes the generation of higher oligomeric species and fibrils. We therefore assume that one important mechanism leading to cognitive improvement in this transgenic animal model is the inhibition of Aβ oligomerization. An important question is the fate of the intracerebral antibody-bound Aβ. Winkler et al.29 observed a rapid increase in serum Aβ in contrast to no changes in intracerebral Aβ as well as CSF Aβ levels levels following treatment with monoclonal anti-Aβ antibodies. Of note, Winkler et al.29 suggested a peripheral mode of action by antibody binding to serum Aβ and subsequent stabilization. Though, Winkler et al.29 could also observe a strong cerebral amyloid-plaque binding following infusion with anti-Aβ antibodies. Interestingly, we could also detect an effect of nAbs-Aβ on intracerebral Aβ. In addition to a sequestration of Aβ in plasma referring to the peripheral sink hypothesis as postulated by DeMattos et al.30, 31 our study points towards a penetration of antibodies into the CNS and a direct intracerebral activity. This is also in line with the aforementioned study by Yamada et al.,24 and our own study showing a cerebral accumulation of human nAbs-Aβ in the APP23 model.32 An analysis of serum Aβ was not part of this study. To address the question of the fate of the increased brain monomeric Aβ following treatment with nAbs-Aβ an analysis of serum levels should be performed in a further study.
Furthermore, we analyzed the influence of nAbs-Aβ on cytokine concentrations in Tg2576 mice. Compared with WT littermates, there was an increase in the proinflammatory cytokines IL-1, IL-6, TNF-α and IFN-γ in transgenic animals. nAbs-Aβ treatment led to a reduction in cytokine release in our animal model of AD. Proinflammatory cytokines are known to have an important role in AD.33 Therefore, it is most likely that cytokines are important in the maintenance of a homeostatic environment and required for proper functioning of complex neuronal circuits. Interestingly, cytokine concentrations, localization as well as timing of its secretion seem to be of importance.34, 35 Our data show a reduction of cytokine concentration in APP transgenic mice similar to those seen in WT littermates. It seems likely that nAbs-Aβ therapy helps to restore the physiological cytokine environment. We argue that inhibition of Aβ oligomerization is partially responsible for this effect. However, a direct effect, of nAbs-Aβ on proinflammatory cytokine levels cannot be ruled out. Luo et al.36 could show a direct effect of intravenous Ig on intracerebral IL-6 and interferon-γ levels in a mouse model of epilepsy.
It is known that Aβ oligomers lead to synaptic dysfunction.37 Recently, it was reported that a single dose of passive immunization against Aβ led to a remarkable improvement of synaptic structures within hours, and that this effect could last for 30 days.38 We observed a modulation of synaptic plasticity following administration of nAbs-Aβ. There are several explanations for the described improvement in synaptic plasticity. Our previous study showed a strong binding of nAbs-Aβ to Aβ oligomers and a protective effect towards Aβ-oligomer induced neurotoxicity. A prevention of Aβ-dependent impaired synaptic transmission by antioligomeric antibodies has been recently reported by Hillen et al.39 Additionally, nAbs-Aβ prevent the generation of oligomers by inhibiting aggregation and could subsequently reduce a synaptotoxic influence of Aβ oligomers. As we could also observe a reduction of intracerebral proinflammatory cytokines in treated animals, this may also contribute to the restoration of synaptic plasticity.
Finally, we observed impaired cognition in Tg2576 mice with deficits in object location memory; acute passive immunization with nAbs-Aβ reversed spatial memory impairments in Tg2576 mice. A number of studies are published showing beneficial effects of active and passive immunization in APP transgenic mouse models for AD using different spatial learning and memory tests (for review, see Röskam et al.7). A range of passive immunization strategies were found to be as effective as active vaccination on spatial learning and memory experiments.40 Treatment with 2286 (a mouse monoclonal antihuman Aβ28–40 antibody) in Tg2576 mice reversed impairments in the radial arm water maze test.41 Triple transgenic TgAD mice immunized with a monoclonal Aβ1-17 antibody (1560) or 20.1 (a monoclonal Aβ1-8 antibody) were found to ameliorate deficits seen in the T-maze and MWM tests.42, 43
In summary, we provide further evidence for a potential role of naturally occurring antibodies in the context of AD. The significant therapeutic effect of an acute passive immunotherapy with nAbs-Aβ on object location memory in old-aged Tg2576 mice one day before testing is based on several mechanisms. Reversal of synaptic dysfunction and subsequent restoration of synaptic communication in the neural systems, modulation of proinflammatory cytokine release as well as a reduction of toxic oligomers are probably the main mechanisms responsible for the observed cognitive improvements in Tg2576 mice.
Early studies by our group have demonstrated the effective use of IVIg, which contain nAbs-Aβ, in patients with AD.44 Similar results were obtained by Relkin et al.12 Therefore, isolation of naturally occurring antibodies from IVIg preparations is a first step in order to prove their efficacy. In a second step, it is essential to understand their physiological role, so that finally, these can be heterologously expressed or chemically synthesized in a large scale. Further research is therefore required to investigate the role of nAbs-Aβ, but we consider their high potential in the treatment of AD.
References
Selkoe DJ . Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 2001; 81: 741–766.
Walsh DM, Selkoe DJ . Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 2004; 44: 181–193.
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ . Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol 2006; 572 (Pt 2): 477–492.
Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med 2005; 11: 556–561.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999; 400: 173–177.
Brody DL, Holtzman DM . Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci 2008; 31: 175–193.
Röskam S, Neff F, Schwarting R, Bacher M, Dodel R . APP transgenic mice: the effect of active and passive immunotherapy in cognitive tasks. Neurosci Biobehav Rev 2010; 34: 487–499.
Britschgi M, Olin CE, Johns HT, Takeda-Uchimura Y, LeMieux MC, Rufibach K et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc Natl Acad Sci USA 2009; 106: 12145–12150.
Dodel R, Hampel H, Depboylu C, Lin S, Gao F, Schock S et al. Human antibodies against amyloid beta peptide: a potential treatment for Alzheimer’s disease. Ann Neurol 2002; 52: 253–256.
Du Y, Wei X, Dodel R, Sommer N, Hampel H, Gao F et al. Human anti-beta-amyloid antibodies block beta-amyloid fibril formation and prevent beta-amyloid-induced neurotoxicity. Brain 2003; 126 (Pt 9): 1935–1939.
Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Förster S et al. Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer's disease: a phase 2, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol 2013 doi:pii: S1474-4422(13)70014-0. 10.1016/S1474-4422(13)70014-0.
Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 2009; 30: 1728–1736.
Kellner A, Matschke J, Bernreuther C, Moch H, Ferrer I, Glatzel M . Autoantibodies against beta-amyloid are common in Alzheimer’s disease and help control plaque burden. Ann Neurol 2009; 65: 24–31.
Dodel R, Balakrishnan K, Keyvani K, Deuster O, Neff F, Andrei-Selmer L-C et al. Naturally occurring autoantibodies against ß-amyloid: investigating their role in transgenic animal and in-vitro models of Alzheimer’s disease. J Neurosci 2011; 31: 5847–5854.
Du Y, Dodel R, Hampel H, Buerger K, Lin S, Eastwood B et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology 2001; 57: 801–805.
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003; 300: 486–489.
Klafki HW, Wiltfang J, Staufenbiel M . Electrophoretic separation of betaA4 peptides (1-40) and (1-42). Anal Biochem 1996; 237: 24–29.
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 1996; 274: 99–102.
Hale G, Good M . Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgments in adult mice expressing the APPswe Alzheimer’s disease mutation. Behav Neurosci 2005; 119: 884–891.
Liu L, Duff K . A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp 2008 pii: 960.
Harris KM, Cruce WL, Greenough WT, Teyler TJA . Golgi impregnation technique for thin brain slices maintained in vitro. J Neurosci Methods 1980; 2: 363–371.
Glabe CG . Structural classification of toxic amyloid oligomers. J Biol Chem 2008; 283: 29639–29643.
McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat Med 2002; 8: 1263–1269.
Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci 2009; 29: 11393–11398.
Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain 2010; 133 (Pt 5): 1328–1341.
O’Nuallain B, Klyubin I, Mc Donald JM, Foster JS, Welzel A, Barry A et al. A monoclonal antibody against synthetic Abeta dimer assemblies neutralizes brain-derived synaptic plasticity-disrupting Abeta. J Neurochem 2011; 119: 189–201.
Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006; 440: 352–357.
Sherman MA, Lesne SE . Detecting abeta*56 oligomers in brain tissues. Methods Mol Biol 2011; 670: 45–56.
Winkler DT, Abramowski D, Danner S, Zurini M, Paganetti P, Tolnay M et al. Rapid cerebral amyloid binding by Abeta antibodies infused into beta-amyloid precursor protein transgenic mice. Biol Psychiatry 2010; 68: 971–974.
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM . Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2001; 98: 8850–8855.
DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM . Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 2002; 295: 2264–2267.
Bacher M, Depboylu C, Du Y, Noelker C, Oertel WH, Behr T et al. Peripheral and central biodistribution of (111)In-labeled anti-beta-amyloid autoantibodies in a transgenic mouse model of Alzheimer’s disease. Neurosci Lett 2009; 449: 240–245.
Steinman L . Nuanced roles of cytokines in three major human brain disorders. J Clin Invest 2008; 118: 3557–3563.
Figiel I . Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiol Exp (Wars) 2008; 68: 526–534.
Ji C, Song C, Zuo P . The mechanism of memory impairment induced by abeta chronic administration involves imbalance between cytokines and neurotrophins in the rat hippocampus. Current Alzheimer research 2011; 8: 410–420.
Luo X, Li D, Cen D, He Z, Meng Z, Liang L . Effect of intravenous immunoglobulin treatment on brain interferon-gamma and interleukin-6 levels in a rat kindling model. Epilepsy Res 2010; 88: 162–167.
Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 2000; 20: 4050–4058.
Spires-Jones TL, Mielke ML, Rozkalne A, Meyer-Luehmann M, de Calignon A, Bacskai BJ et al. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. NeurobiolDis 2009; 33: 213–220.
Hillen H, Barghorn S, Striebinger A, Labkovsky B, Muller R, Nimmrich V et al. Generation and therapeutic efficacy of highly oligomer-specific beta-amyloid antibodies. J Neurosci 2010; 30: 10369–10379.
Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT et al. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci 2002; 22: 6331–6335.
Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation 2004; 1: 24.
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM . Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005; 45: 675–688.
Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM . Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem 2006; 281: 39413–39423.
Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75: 1472–1474.
Acknowledgements
We would like to thank Christine Forbach, Tanja Rausch and Carola Gäckler for excellent technical support. We would like to extend our thanks to Dr Jens-Peter Reese for his help with statistical analysis. In addition, we thank Dr Kayed and Dr Du for kindly providing the anti-Aβ antibodies A11 and 3D6, respectively. This work was in part financed by a research grant from Grifols awarded to JPB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Bacher, Dr Balakrishnan, Dr Dodel hold patents in respect to naturally occurring autoantbodies. All other authors have no conflict of interest concerning the contents of the article.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mengel, D., Röskam, S., Neff, F. et al. Naturally occurring autoantibodies interfere with β-amyloid metabolism and improve cognition in a transgenic mouse model of Alzheimer’s disease 24 h after single treatment. Transl Psychiatry 3, e236 (2013). https://doi.org/10.1038/tp.2012.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2012.151
Keywords
This article is cited by
-
A combination of multiple autoantibodies is associated with the risk of Alzheimer’s disease and cognitive impairment
Scientific Reports (2022)
-
Naturally-Occurring Antibodies Against Bim are Decreased in Alzheimer’s Disease and Attenuate AD-type Pathology in a Mouse Model
Neuroscience Bulletin (2022)
-
Immunological memory to hyperphosphorylated tau in asymptomatic individuals
Acta Neuropathologica (2017)
-
Reduced β-amyloid pathology in an APP transgenic mouse model of Alzheimer’s disease lacking functional B and T cells
Acta Neuropathologica Communications (2015)
-
Passive anti-amyloid immunotherapy in Alzheimer's disease: What are the most promising targets?
Immunity & Ageing (2013)