Abstract
Previous studies suggested that the reactive hyperemia index (RHI) is a promising cardiovascular risk predictor. We aimed to evaluate clinical determinants of RHI and its association with circulating endothelial injury and cardiac markers in hemodialysis patients. Among 368 patients recruited, RHI was evaluated by peripheral arterial tonometry (PAT) on a midweek nondialysis day. Clinical determinants of RHI were explored by multiple stepwise regression analysis and associations between RHI and circulating markers were evaluated by general linear models. The major cause of a failed PAT test was poor signal (82.1%). Intraclass correlation coefficient for reproducibility evaluation was 0.74. Multiple regression analysis showed traditional clinical factors only explained 7% of the variance of natural logarithm RHI (LnRHI) in the patients. In association analyses, LnRHI showed significant positive associations with Von Willebrand factor (vWF) (p = 0.04) and tissue factor (p = 0.047). It also associated positively with troponins (p ≤ 0.02 for both). In conclusion, performance of the PAT test was acceptable in dialysis patients and traditional clinical variables had very limited influence on RHI in these subjects. Among a panel of conventional endothelial injury markers, RHI showed very modest associations with only vWF and tissue factor. RHI associated positively with troponins in the patients.
Similar content being viewed by others
Introduction
Patients with kidney disease suffer from an increased risk of cardiovascular events as renal function declines1. For those on maintenance hemodialysis, cardiovascular disease remains the leading cause of death2. This high prevalence and severity of cardiovascular injury in these patients is believed to be induced by both traditional and non-traditional risk factors, of which endothelial dysfunction is suggested to play an important role3. Endothelial dysfunction is a dominant feature of atherogenesis and a key aspect in the development of cardiovascular diseases. It is characterized primarily by reduced bioactivity of nitric oxide (NO), which is essential to the regulation of vascular tone and homeostasis4.
In clinical practice and research studies, the most common technique to evaluate endothelial function is flow-mediated vasodilatation (FMD) of the brachial artery. Previous studies have demonstrated that endothelial function evaluated by this method correlate closely with results measured directly in the coronary artery5,6. However, its application in practice requires a highly experienced operator, and comparability in different settings is challenging7. Recently, a new device (EndoPAT 2000) using peripheral arterial tonometry (PAT) provides an alternative option for non-invasive measurement of endothelial function. Placed on a fingertip, the device measures pulse arterial volume changes induced by upper arm cuff occlusion and generates the reactive hyperemia index (RHI) automatically. Unlike the FMD measurement, RHI is operator-independent and easy to perform. Several studies have validated its value in assessing coronary microvascular endothelial function and in predicting cardiovascular outcome in patients at risk, including those with chronic kidney disease8,9,10.
A previous study has adopted the index as marker of endothelial function in a group of peritoneal dialysis patients11. However, there is a paucity of data regarding RHI in patients on hemodialysis, in spite of the fact that these patients bear a significant burden of cardiovascular disease. Exploring non-traditional and novel risk indicators is of great importance for these individuals. In addition, the presence of an arteriovenous fistula in these patients may influence the test results as well as its clinical relevance. Here, we explored the performance of the test in dialysis patients to evaluate its clinical utility with a focus on: 1). clinical determinants of this index and 2). its associations with traditional endothelial injury markers and cardiac risk markers.
Subjects and Methods
Study Population
We performed this cross-sectional analysis using baseline data from an ongoing prospective cohort study, which aims to study vascular dysfunction in dialysis patients. The study included patients on maintenance hemodialysis (four hours per treatment and thrice weekly over 3 months) and aged 18–80 years. All patients reached their dry weight based on clinical judgement. Exclusion criteria included: 1. Malignant hypertension with systolic blood pressure (SBP) ≥180 mmHg or diastolic blood pressure (DBP) ≥110 mmHg; 2. Acute infection, acute heart failure or the acute phase of stroke; 3. Decompensated liver cirrhosis or malignant tumor; 4. Amyloidosis or dilated cardiomyopathy; 5. Internal jugular venous catheterization, previous fistula creation on the non-access arm or any other conditions that will affect the accuracy of peripheral arterial tonometry. Coordinating centers of the study included dialysis departments of six tertiary hospitals: The Second Affiliated Hospital of Nanjing Medical University (Nanjing, China), The Third Affiliated Hospital of Soochow University (Changzhou, China), Luan People’s Hospital (Luan, China), Affiliated Yixing People’s Hospital of Jiangsu University (Yixing, China), Affiliated Wuxi People’s Hospital of Nanjing Medical University (Wuxi, China), Yijishan Hospital of Wannan Medical College (Wuhu, China). Sixty age and sex-matched healthy controls (without any known history of chronic kidney disease or cardiovascular disease) were also recruited to assess the difference of RHI between patients and healthy subjects. The study was approved by the Institutional Ethical Committee of The Second Affiliated Hospital of Nanjing Medical University. The study was conducted in accordance with approved guidelines and all participants provided written informed consents.
Peripheral Arterial Tonometry Measurement
PAT measurement was performed on a midweek nondialysis day. Patients were instructed to abstain from meals, caffeine, and nitrates within 2 hours (long-acting nitrates for 12 hours) before the measurement. Test room temperature was set at 21–25 °C. Peripheral blood pressure was measured at least 20 minutes before the test was started. The EndoPAT 2000 (Itamar Medical Inc., Israel) device was used for the measurement. Specially designed finger probes were placed on the index finger of each hand. Baseline measurement lasted for 6 minutes and followed by occlusion of pulsatile arterial flow for 5 minutes. Occlusion was done through inflation of a blood pressure cuff on the non-access arm, started at a pressure of 250 mmHg and increased until a complete occlusion was achieved as judged by the PAT signal or to a maximum of 300 mmHg. After occlusion, PAT signal was recorded for another 5 minutes. Results were analyzed automatically using an algorithm by the computer12. For reproducibility evaluation, a repeated test was performed on the same time of the following week.
Blood Pressure Measurements
Blood pressure (BP) level was determined by ambulatory blood pressure (ABP) monitoring, which started on the midweek nondialysis day and ended before next dialysis treatment. The SpaceLabs 90217 (SpaceLabs Medical Inc, Redmond, WA) monitor was used and programmed to measure BP every 20 minutes during the day-time (6:00 am.–10:00 pm.) and every 30 minutes during the night-time (10:00 pm.–6:00 am.). Patients were instructed to follow their daily activity and keep their arm immobile during measurements. Recordings were downloaded using manufacturer’s software (SpaceLabs Report Manager System) and were further extracted and analyzed by SPSS 19.0 (SPSS Inc, Chicago, IL). For patients who had <6 ABP readings, their predialysis blood pressures from dialysis records were collected and averaged over 2-weeks (6 times) before the ABP measurements and were used instead of ABP results.
Laboratory Tests and Biomarker Measurements
Blood samples were drawn through vascular access before dialysis treatment. Routine laboratory tests were performed in each coordinating center. Plasma samples were stored at −80 °C until needed for testing.
Plasma biomarkers, including brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT proBNP), Troponin I, Troponin T and C-reactive protein (CRP), plasminogen activator inhibitor-1 (PAI-1), Von Willebrand factor (vWF), sE-Selectin, tissue factor, thrombomodulin were measured using the Milliplex Map assays (Merck Millipore, Shanghai, China). Concentrations were calculated using the 5-parameter logistic curve fit. Results outside the range of the standards or the fit were further analyzed via cubic spline.
Statistical Analysis
Data were expressed as mean ± standard deviations or median (interquartile range) for numerical variables and counts (%) for categorical variables. Numerical variables with skewed distribution were logarithm transformed. RHI was expressed in the form of its natural logarithm: LnRHI. Comparisons between two groups were done using the Student’s t test or chi-square test as appropriate. Pearson’s correlation coefficient was used for univariate association analysis and variables with p < 0.10 were selected and entered into the following multiple stepwise regression model. Associations between RHI and plasma markers were determined by general linear models. Three models for the association analysis were constructed with different adjustments. The Model-1 was a basic model in which only age and sex were adjusted. In Model-2, major cardiovascular risk factors were included: age, sex, body mass index, smoking status, diabetes mellitus, history of cardiovascular disease, use of antihypertensives, use of statins, total and HDL cholesterol, triglycerides and mean arterial pressure. In Model-3, dialysis-specific risk factors (dialysis vintage and interdialytic weight gain) and log-transformed CRP were added as covariates. All statistical analyses were performed using SPSS 19.0 (IBM SPSS, Chicago, IL). A p value < 0.05 was considered statistically significant.
Results
General Test Performance and Reproducibility
A total of 368 patients were recruited between July 2015 and July 2016. Eighteen of them did not undergo PAT test due to device availability. Among those who underwent the test, PAT results were available for 311 patients. Causes of a failed test included: poor PAT signal (n = 32, 82.1%), signal with too much noise (n = 1, 2.6%), incomplete occlusion (n = 3, 7.7%), computer breakdown (n = 1, 2.6%), intolerance of occlusion (n = 1, 2.6%) and hand abnormality (n = 1, 2.6%). Representative data from successful and unsuccessful tests are presented in Fig. 1.
Twenty patients were invited to assess reproducibility of testing. Two failed the first test and another two failed the second test. Sixteen had successful first and second tests and the intraclass correlation coefficient was 0.74.
Characteristics of Patients according to LnRHI Category
General clinical information of the 311 patients who had successful PAT test is presented in Table 1. Mean age of the study population was 52.6 years and 175 (56.3%) were male. Patients with a higher LnRHI had lower body mass index (BMI), lower total cholesterol and triglyceride level. Blood pressure level was significantly lower in patients with reduced LnRHI. There was no significant difference regarding other parameters.
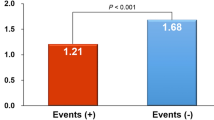
LnRHI was also compared between patients and age, sex-matched healthy controls. Although LnRHI tended to be lower in the patients than in the control subjects, the difference did not reach statistical significance (Patients: 0.52 ± 0.34; Controls: 0.59 ± 0.29; p = 0.14) (Table 2).
Independent Clinical Determinants of LnRHI
In univariate analysis, LnRHI positively associated with phosphorus, SBP, DBP and negatively associated with triglyceride and calcium. These variables were further analyzed by multiple stepwise regression analysis and only DBP (ß = 0.006, p < 0.001) and calcium (ß = −0.183, p = 0.01) remained as independent determinants of LnRHI. The model explained 7% of the variance of LnRHI (Table 3).
Associations between LnRHI and Conventional Endothelial Injury Markers
Table 4 presented the associations between LnRHI and conventional endothelial injury markers. In Model-1 with only age and sex adjusted, LnRHI showed no association with these markers except for tissue factor (ß = 0.12, p = 0.01). After adjusting for traditional cardiovascular risk factors in Model-2, the association between LnRHI and tissue factor became non-significant, with a marginal p value of 0.06. In the extensively adjusted Model-3 with dialysis vintage, interdialytic weight gain and log-CRP included as covariates, LnRHI was positively associated with vWF (ß = 0.09, p = 0.04) and tissue factor (ß = 0.09, p = 0.047).
Associations between LnRHI and Cardiac Markers
Table 5 showed the associations between LnRHI and cardiac markers. There was a significant positive association between LnRHI and log-BNP in Model-1. However, after extensive adjustment (Model-2 and 3), the association disappeared. LnRHI showed no association with log-NT proBNP in any model. On the contrary, it was closely and positively associated with troponin levels in all three models.
Discussion
Identifying novel cardiovascular risk markers is of critical importance for dialysis patients. Considering previous evidence from a variety of study populations8,9,10, it was natural to hypothesize that RHI is also a promising cardiovascular risk predictor in dialysis patients. To evaluate its value in this unique disease population, we performed this cross-sectional analysis with a focus on its performance and its cross-sectional correlators.
The existence of arteriovenous fistula access poses a special challenge for the performance of PAT test among dialysis patients. Obviously, the arm with the fistula should only be used as a control arm to avoid occlusion by the blood pressure cuff. Since a previous history of fistula-associated operation, even a failed one, could affect distal blood perfusion, we excluded those with previous operations for fistula creation on the non-access arm. However, even the fistula on the control arm could also have had unneglectable influences on the test result. First, fistula creation decreases digital perfusion pressure, thus may result in insufficient PAT signal13, as reflected by the high incidence of poor signal quality in our study. Second, the fistula may cause unsteady blood flow, e.g. turbulent flow, in the local circulation which can disrupt the steady PAT signal of the control arm and thus affect the calculation of RHI. Finally, the control arm serves as reflection of systemic state, but existence of the fistula may blunt the transduction of systemic fluctuation into distal circulation. Caution should be taken in interpreting our data since these probable influences cannot be avoided in the patients.
Unlike the general population, BP measurement before PAT test had to be done in the test arm. To avoid the affection of transient induction of NO release on the subsequent reactive hyperemia test, we stipulated that the test could only be performed no earlier than 20 minutes after BP measurement. Under these special restrictions, the test was successful in 88.9% subjects (311 out of 350). The main cause of a failed test was poor PAT signal, accounting for over 80% of the technically inadequate studies. This ratio was significantly higher than that reported in the Framingham cohort (30.9%) and the ELSA-Brasil cohort (54.0%)14,15. Therefore, signal quality is the major limitation of a successful PAT test in dialysis patients, even with strict eligibility restrictions. Among those with a successful test, the intraclass correlation coefficient analysis indicates that the reproducibility was moderate. Hence, the overall performance of the PAT test in dialysis patients is acceptable.
Unexpectedly, we found no statistically significant difference of LnRHI between dialysis patients and the control population, even though we noted that it tended to be lower in the dialysis patients. In a previous study by Hirata et al., the authors found a significantly lower LnRHI in CKD-patients (n = 383) compared with non-CKD patients (n = 474) (0.525 ± 0.194 vs. 0.588 ± 0.216, p < 0.001)10. It is worthy to note that the average LnRHI levels in their study were similar to ours, for both the patients and the controls. Therefore, a possible explanation for the absence of significant difference between patients and controls in our study is that we had limited number of the control subjects (n = 58).
Previous studies suggested that compared with FMD, RHI correlates more closely with metabolic factors14,16. In our analysis, this was partly confirmed as we found that dialysis patients with higher LnRHI tended to have lower BMI, lower total cholesterol and triglyceride levels. In addition, the linear regression analysis revealed that DBP (positively) and calcium (negatively) were independent determinants of LnRHI. However, it should be noted that the R2 of the model was only 0.07 and the regression coefficient for DBP is particularly low, suggesting that the associations were essentially clinically non-significant, and that this index is largely determined by unknown non-traditional clinical variables. In fact, studies on the determinants of RHI yielded widely varied and sometimes conflicting results and the abilities of their regression models for variance predicting were usually unsatisfied14,15,17,18,19,20. Hence more potent determinants of RHI, especially disease-specific factors, warrant further exploring.
The associations of RHI with several conventional endothelial injury markers were also evaluated in our study. We found that LnRHI had a modest association with vWF and tissue factor after extensive adjustment. These markers were widely used in different studies as surrogate measures of endothelial dysfunction. However, our results, together with previous studies, suggest that these markers and non-invasive endothelial function measures (both FMD and RHI) should not be regarded as interchangeable21,22.
Our analysis also revealed a paradoxically positive association between RHI and troponins. Elevated troponin levels are powerful prognostic predictors in dialysis patients23. Whether this indicates another “reverse epidemiology” phenomenon or implies that RHI and the markers respond to distinct pathophysiology process is unknown. In fact, confusing association between RHI and another cardiovascular risk marker, carotid artery intima-media thickness, has also been observed in the ELSA-Brasil cohort24. Future studies, especially those with hard outcomes, should pay attention to this question.
There are certain limitations in our study that should be noted. First, due to the cross-sectional design, we cannot establish any causality conclusions. Second, the exact effect of different dialysis access (type and location) on the PAT signal and RHI was not determined in our study, though we observed that poor signal was the major cause of a test failure. Third, although patients underwent repeated PAT testing on the same midweek nondialysis day during consecutive weeks, we did not obtain any measurement about their fluid status. Therefore the exact effect of volume status on RHI cannot be extrapolated from our study.
In conclusion, poor signal was the major cause of failed PAT test in dialysis patients. Traditional clinical variables had very limited influence on RHI in these subjects. Among a panel of conventional endothelial injury markers, RHI showed very modest associations with only vWF and tissue factor. RHI was positively associated with troponins in the patients. Our results showed that PAT testing is feasible in patients on hemodialysis. Future studies are warranted to explore the risk discrimination ability of RHI in these patients.
Additional Information
How to cite this article: Wenjin, L. et al. Reactive Hyperemia Index in Patients on Maintenance Hemodialysis: Cross-sectional Data from a Cohort Study. Sci. Rep. 7, 45757; doi: 10.1038/srep45757 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine 351, 1296–1305, doi: 10.1056/NEJMoa041031 (2004).
de Jager, D. J. et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. Jama 302, 1782–1789, doi: 10.1001/jama.2009.1488 (2009).
Stenvinkel, P. et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clinical journal of the American Society of Nephrology: CJASN 3, 505–521, doi: 10.2215/CJN.03670807 (2008).
Gutierrez, E. et al. Endothelial dysfunction over the course of coronary artery disease. European heart journal 34, 3175–3181, doi: 10.1093/eurheartj/eht351 (2013).
Anderson, T. J. et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology 26, 1235–1241 (1995).
Takase, B. et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. The American journal of cardiology 82, 1535–1539, A1537–1538 (1998).
Thijssen, D. H. et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology. Heart and circulatory physiology 300, H2–12, doi: 10.1152/ajpheart.00471.2010 (2011).
Bonetti, P. O. et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology 44, 2137–2141, doi: 10.1016/j.jacc.2004.08.062 (2004).
Rubinshtein, R. et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal 31, 1142–1148, doi: 10.1093/eurheartj/ehq010 (2010).
Hirata, Y. et al. Endothelial function and cardiovascular events in chronic kidney disease. International journal of cardiology 173, 481–486, doi: 10.1016/j.ijcard.2014.03.085 (2014).
Sarmento-Dias, M. et al. Fibroblast growth factor 23 is associated with left ventricular hypertrophy, not with uremic vasculopathy in peritoneal dialysis patients. Clinical nephrology 85, 135–141, doi: 10.5414/CN108716 (2016).
Azuma, M. et al. Association between endothelial function (assessed on reactive hyperemia peripheral arterial tonometry) and obstructive sleep apnea, visceral fat accumulation, and serum adiponectin. Circulation journal: official journal of the Japanese Circulation Society 79, 1381–1389, doi: 10.1253/circj.CJ-14-1303 (2015).
Martin, A. G., Grasty, M. & Lear, P. A. Haemodynamics of brachial arteriovenous fistula development. The journal of vascular access 1, 54–59 (2000).
Hamburg, N. M. et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 117, 2467–2474, doi: 10.1161/CIRCULATIONAHA.107.748574 (2008).
Brant, L. C., Hamburg, N. M., Barreto, S. M., Benjamin, E. J. & Ribeiro, A. L. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort study. Journal of the American Heart Association 3, e001279, doi: 10.1161/JAHA.114.001279 (2014).
Benjamin, E. J. et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 109, 613–619, doi: 10.1161/01.CIR.0000112565.60887.1E (2004).
Ertek, S. et al. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Archives of medical science: AMS 8, 47–52, doi: 10.5114/aoms.2012.27280 (2012).
Kelly, A. S., Marlatt, K. L., Steinberger, J. & Dengel, D. R. Younger age is associated with lower reactive hyperemic index but not lower flow-mediated dilation among children and adolescents. Atherosclerosis 234, 410–414, doi: 10.1016/j.atherosclerosis.2014.03.031 (2014).
Piglowska, M. et al. Body composition, nutritional status, and endothelial function in physically active men without metabolic syndrome–a 25 year cohort study. Lipids in health and disease 15, 84, doi: 10.1186/s12944-016-0249-9 (2016).
Torimoto, K., Okada, Y., Mori, H. & Tanaka, Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovascular diabetology 12, 1, doi: 10.1186/1475-2840-12-1 (2013).
Chen, Y. et al. High levels of soluble intercellular adhesion molecule-1, insulin resistance and saturated fatty acids are associated with endothelial dysfunction in healthy adolescents. Atherosclerosis 211, 638–642, doi: 10.1016/j.atherosclerosis.2010.03.013 (2010).
Solini, A. et al. Adipocytokine levels mark endothelial function in normotensive individuals. Cardiovascular diabetology 11, 103, doi: 10.1186/1475-2840-11-103 (2012).
Wang, A. Y. & Wai-Kei Lam, C. The diagnostic utility of cardiac biomarkers in dialysis patients. Seminars in dialysis 25, 388–396, doi: 10.1111/j.1525-139X.2012.01099.x (2012).
Lemos, S. P. et al. Inconsistent Correlation Between Carotid Artery Intima-Media Thickness and Peripheral Arterial Tonometry: Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Medicine 94, e1403, doi: 10.1097/MD.0000000000001403 (2015).
Acknowledgements
We are grateful to the patients who participated in our study. We thank Dr. Roderick Tan for his contribution in language editing. Ms Nie Ruimin is thanked for her technical support for biomarker measurement. This study was supported by a grant for clinical research from Jiangsu Science and Technology Department to Dr. Junwei Yang (Grant Number: BL2013037).
Author information
Authors and Affiliations
Contributions
Research idea and study design: Y.J., L.W.; data acquisition: L.W., M.M., W.L., S.Z., L.X., Z.J., G.C., Z.J., C.H., F.W., B.Y.W.; data analysis/interpretation: L.W., Y.J.; statistical analysis: C.J.; supervision or mentorship: Y.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, W., Meng, M., Chen, J. et al. Reactive Hyperemia Index in Patients on Maintenance Hemodialysis: Cross-sectional Data from a Cohort Study. Sci Rep 7, 45757 (2017). https://doi.org/10.1038/srep45757
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45757
This article is cited by
-
Consequences of Supraphysiological Dialysate Magnesium on Arterial Stiffness, Hemodynamic Profile, and Endothelial Function in Hemodialysis: A Randomized Crossover Study Followed by a Non-Controlled Follow-Up Phase
Advances in Therapy (2020)
-
Relationship between serum uric acid level and vascular injury markers in hemodialysis patients
International Urology and Nephrology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.