Abstract
As well known, signet-ring cell carcinoma (SRCC) is a rare histological subtype of colorectal adenocarcinoma, which has been associated with poor prognosis and resistant to non-surgery therapy compared with common adenocarcinoma. In this study, we assessed the effect of preoperative radiotherapy (PRT) for locally advanced rectal SRCC in a large patient group from the Surveillance, Epidemiology, and End Results program (SEER, 1988–2011) database. SRCC was found in 0.9% (n = 622) rectal cancer (RC) patients in our study. In the PRT setting, SRCC had significantly worse cancer-specific survival than mucinous adenocarcinoma and nonmucinous adenocarcinoma patients (log-rank, P < 0.001). In terms of SRCC, stage III RC patients benefited from PRT (log-rank, P < 0.001) while stage II did not (P = 0.095). The multivariate Cox proportional hazard model showed that PRT was an independent benefit factor in stage III rectal SRCC patients (HR, 0.611; 95% CI, 0.407–0.919; P = 0.018). In conclusion, SRCC was an independent predictor of poor prognosis in stage III RC patients, but not in stage II. In the PRT setting of locally advanced RC, SRCC patients had significantly worse prognosis. PRT was an independent prognostic factor associated with improved survival in stage III rectal SRCC.
Similar content being viewed by others
Introduction
As a major health burden worldwide, colorectal cancer (CRC) remains the second leading cause of cancer related mortality in the United States1,2. While adenocarcinomas are the most common tumours of the colon and rectum, variant histological subtypes have been reported to be associated with varied clinical feature and survival. As a rare histology subtype of adenocarcinoma, signet-ring cell carcinoma (SRCC) is defined by the World Health Organization (WHO) containing abundant intracellular mucin in more than 50% of its cells3,4,5. SRCC is found in 0.1–2.6% of CRC patients6,7. However, there are obvious regional differences. In some countries, such as Jordan and Lebanon, the frequency of SRCC is reported to reach 18.5%8. On the other hand, the frequency is only 1% in United States9. SRCC is recognized to constitute a distinct pathological entity within the spectrum of CRCs. There are several consistent findings focused on differences among SRCC, mucinous adenocarcinoma (MC) and nonmucinous adenocarcinoma (NMC) in the colorectum. They have demonstrated that SRCC was related to younger age at diagnosis, more advanced stage at presentation and a poorer survival compared with MC and NMC4,5,10,11. SRCC presented extensive lymphatic spread, more frequently with multiple metastatic sites and high risk of peritoneal metastases12. Some studies have reported molecular and genetic differences among SRCC, MC and NMC, contributing to a more aggressive biological behaviour13.
The treatment of rectal cancer (RC) patients has improved rapidly in recent years. Many oncologists considered RC patients with stage II–III as an indication for preoperative radiotherapy (PRT), and recommend to add chemotherapy for patients with locally advanced RC in which the mesorectal fascia is threatened4,14,15. The efficacy of neo-adjuvant therapy including PRT for RC has been described in many literatures16,17,18. SRCC was prone to extensive lymphatic spread and peritoneal metastases leading to poor survival. However, the PRT concerning rectal SRCC patients, which is limited to a low incidence, has been rarely reported17,19.
In this study, we analysed the clinicopathological characteristics of SRCC and established the prognostic implication of SRCC on locally advanced RC patients who were treated with PRT. Then we determined whether or not locally advanced rectal SRCC patients benefit from PRT.
Results
Patient characteristics
A total of 69,543 RC patients were included in this study and most patients were diagnosed with NMC (n = 63,036, 90.6%). MC (n = 5,885) and SRCC (n = 622) were found in 8.5% and 0.9% of patients, respectively (Table 1). The SRCC group presented a younger diagnosis age (59.4 years) than that of NMC (65.4 years) and the MC (65.4 years) (P < 0.001). SRCC patients presented more frequently with stage III tumours than MC and NMC patients (79.4 vs. 57.1%, 52.8%, P < 0.001, respectively) and poorer differentiation (P < 0.001). Compared with RC patients in MC (40.9%) and NMC (35.1%) group, the patients in SRCC (51.6%) group were more likely to be assigned for PRT (P < 0.001).
Prognostic factors in stage II and III RC patients
SRCC patients presented poorer 5 year survival than MC and NMC patients in both of stage II (43.60% vs. 66.28% and 73.10%, P < 0.001, respectively) and III RC (34.55% vs. 53.90% and 63.10%, P < 0.001, respectively). The result of multivariate survival analysis using Cox model concerning all stage II and III RC patients is showed in Table 2. From our analysis, the higher age (>65 years), the larger tumour size (>5 cm), and poorly differentiated tumour grade were all significant factors that worsened survival in stage II and III RC (P < 0.001, respectively). On the other hand, multiple tumour number and more number of lymph nodes examined (NO ≥ 12) were associated with better survival (P < 0.001, respectively). SRCC was an independent predictor of poor prognosis in stage III (HR, 1.985; 95% CI, 1.607–2.452; P < 0.001), but not in stage II patients (P = 0.136). Stage III patients benefited from PRT (HR, 0.799; 95% CI, 0.748–0.854; P < 0.001), while the stage II did not (P = 0.442).
SRCC as a poor prognostic factor in stage II and III RC patients treated with PRT
There were 7,592 (48.1%) stage II patients and 8,198 (51.9%) stage III patients underwent the PRT. SRCC patients underwent PRT had a statistically significant worse cancer-specific survival (CSS) compared with MC and NMC patients in both stage II and III RC patients (Fig. 1A, B, log-rank, P < 0.001, respectively).
PRT for stage II and III rectal SRCC patients
MC and NMC patients were divided into PRT plus surgery group and surgery alone group. As is shown in the Supplementary Fig. S1A, there was no statistically survival difference between the two group in stage II MC patients (log-rank, P = 0.394). But, PRT plus surgery group had a better CSS than that with surgery alone group in stage III MC patients (Supplementary Fig. S1B), stage II (Supplementary Fig. S2A) and III NMC patients (Supplementary Fig. S2B, log-rank, P < 0.001, respectively).
In 622 rectal SRCC patients underwent surgery, 210 patients received PRT. Excluding 12 patients without survival information, 198 patients (stage II, n = 56; stage III, n = 142) remained for analysis and were divided into PRT plus surgery group and surgery alone group. In stage II SRCC patients, there was no statistically survival difference between PRT plus surgery group and surgery alone group (Fig. 2A, log-rank, P = 0.095). Nevertheless, the stage III SRCC patients treated with PRT plus surgery had a better CSS than that with surgery alone (Fig. 2B, log-rank, P < 0.001). As Table 3 shown, PRT was not a significantly benefit factor in a total of stage II and stage III SRCC patients (HR, 0.911; 95% CI, 0.609–1.362; P = 0.648). The further multivariate survival analysis were stratified by each stage. It demonstrated PRT was an independent prognostic factor associated with better CSS in stage III SRCC patients (Table 4. HR, 0.611; 95% CI, 0.407–0.919; P = 0.018).
(A) Cancer specific survival for stage II rectal SRCC patients treated with or without preoperative radiotherapy. (B) Cancer specific survival for stage III rectal SRCC patients treated with or without preoperative radiotherapy. MC = mucinous adenocarcinoma; NMC = nonmucinous adenocarcinoma; SRCC = signet-ring cell carcinoma; RT = radiotherapy.
Discussion
In this population based study, we analysed 69,543 locally advanced RC patients who were registered in the SEER. 0.9% of our population consisted of SRCC, similar to the numbers reported in previous literatures concerning on all stages CRC5,9,10,11. However, this proportion is much less than that of an Indian research, in which, SRCC comprised about 15.3 percent of stage II and III RC patients4. Many well-recognized SRCC associated features, such as younger diagnosis age, more frequently with advanced tumour, poorer differentiation were confirmed in this study4,5,10,11.
SRCC has been associated with poor prognosis compared with common adenocarcinoma9,20. Prognostic factors analysis stratified by tumour stage is rare in previous literatures. By a univariate analysis, Niek Hugen et al.21 showed a relatively worse survival for SRCC compared with non-SRCC in stage II and III CRC, prominently in stage III. We presented that SRCC was an independent predictor of poor prognosis in stage III but not in stage II RC, which had never been reported before. One of the reasons for the poor outcome in SRCC may be low differentiation at diagnosis, which was related to high risk of vascular invasion and lymph node involvement4,22. Ho-Su Lee and his colleagues20 found that most of the SRCC patients presented with an infiltrative growth pattern, which had been proved to be an independent prognostic factor among stage I-III CRC patients13. Moreover, Vallam et al.4 reported that circumferential resection margin (CRM) positivity rate was higher in SRCC (19%) than that in Non-SRCC (4%). The CRM positivity makes a curative surgery therapy impossible and leads to a high risk of local recurrence and poor survival4,10,11,22. The special metastatic pattern in SRCC may be another explanation for the adverse survival12,23. Signet ring cells usually present as single cell or gather as loose clusters, and disrupt cell-cell adhesion which contributes to the aggressive biological behavior. By breaking the E-cadherin/β-catenin complex and amplification of Bcl-224, signet-ring cells can further reduce cell–cell adhesion, loosen the surrounding structure and spread far away. This may be one of explanations for why SRCCs tend to present peritoneal metastases. These metastases cannot be treated by radical surgery which lead directly to a poor prognosis. The genetic variations had been reported to affect survival of CRC25. Furthermore, these genetic factors including RAS (KRAS and NRAS), BRAF, MMR/MSI status maybe linked to chemoradiotherapy efficacy. We will investigate the relationship between genetic factors and chemoradiotherapy efficacy in SRCC in the future.
Due to the low frequency, SRCC has been rarely studied in a PRT setting. In the previous studies, there are probably biases associated with single-institution reporting and relatively small sizes17,26. To our best knowledge, this is the first study analysed outcome of PRT in rectal SRCC patients with long follow up information from multiple institutions. The present study demonstrated that CSS of SRCC patients was poorer than MC and NMC regardless of stages (both stage II and III) in a PRT setting. As mentioned above, SRCC presents a higher CRM positivity rate and infiltrative growth pattern, which mean higher post-operative residual tumour rates. Therefore, even treated with PRT, the SRCC had high recurrence rate and poor prognosis. Bratland et al.17 studied a cohort of 120 RC patients received PRT including six SRCC patients. It showed that rectal SRCC tend to have extensive mesorectal lymph node metastases and extramesorectal lymph node disease within the pelvic cavity which might be associated with poor PRT response. Some researchers tried to ascribe this poor PRT response to radiation resistance. Hugen N et al.16 found that mutated KRAS as a biomarker was strongly associated with poor response to chemoradiotherapy in MC. Though with a higher rate of microsatellite instability (MSI) in MC, the predictive value of MSI in response to radiotherapy is still controversial27,28,29. Expression of high-mobility group box 1, Paf15 and several microRNAs had been reported to be associated with response to chemoradiotherapy30,31,32,33,34. Unfortunately, neither lymph node dissemination nor molecular phenotype status had been recorded in our study and these analyses could not be performed. Further study is still needed to provide additional insight into the molecular mechanism underlying the pathogenesis of SRCC associated with radiation therapy response.
Some researchers thought these stage II patients with lower risk of local recurrence (clear margins and favorable prognosis features) maybe adequately treated with surgery and adjuvant chemotherapy. Many patients were under-staged by preoperative clinical imaging but subsequently proved to have positive lymph nodes in the surgical specimens. Therefore, the PRT in stage II RC remains controversial. Our research implied that stage II rectal SRCC patients did not receive benefit from PRT. The survival analysis revealed an even worse survival of this subgroup than patients who did not undergo PRT, although the difference was not statistically significant. Therefore, PRT may not have been necessary for these patients. The result should be interpreted caution because of the limited number of patients with stage II rectal SRCC in current study. In order to avoid side effects from unnecessary PRT for stage II RC, further study is needed to establish patients selected criterion to benefit this group. Interestingly, our study showed that PRT was associated with improved survival in stage III rectal SRCC. This was in accordance with the study of Bratland and his colleagues17. They suggested that patients with stage III rectal SRCC, when presenting limited lymph node metastasis, should be offered PRT in a tentatively curative setting. However, what worth mentioning is that PRT with improved survival may result in selected patient groups with significant tumour response and patients presenting with limited lymph node disease, similar views can be found in previous literatures35,36. We suggested that stage III rectal SRCC should be arranged for PRT. It could reduce the tumour volume and may facilitate the tumour resection, block the tumour invasion like lymph nodes metastasis or mesorectal fascia threatened, and reduce the local recurrence rate37.
Including the large number of patients from national population-based data, our study avoided the biases associated with single-institution experiences or limited sample sizes. Due to the nonrandomized nature of SEER, several limitations of current study deserved comment. Firstly, reviewing the individual pathological diagnosis was not feasible in a large population size. Variations in interpretation among pathologists may have led to misclassification. To explore the possible heterogeneity among each registration centre, we compared the proportion of SRCC, and there were no significant differences. Secondly, the SEER registry does not include detail information concerning the dose or duration of chemoradiation including PRT. Therefore, we were not able to take differences in PRT practice into account over the study period. Furthermore, some of the patients in our study may have underwent an emergency surgery due to bowel obstruction, thereby comprising a larger share in the patient group that did not receive PRT. Although data regarding cancer recurrences was not available in present study, cancer-specific survival is a reasonable surrogate of rectal cancer-specific outcome. Despite these, the results of the current research may provide some information for future studies of SRCC in relation to PRT in this area. In order to obtain a more definitive conclusion, further larger randomized controlled trial of Chinese population will be conducted through a multicentre cooperation.
In summary, our results showed that SRCC was a distinct entity that more often affected younger patients, presented more advanced tumour, poorer differentiation at diagnosis compared to non-signet ring cell carcinoma. SRCC remained a poor prognostic factor in locally advanced (stage II and III) RC patients who underwent PRT. Although PRT for stage II rectal SRCC was not associated with improved survival, it was an independent prognostic factor associated with better CSS in stage III SRCC patients. Further study is needed to elucidate rectal SRCC patients’ selection criterion for PRT.
Patients and Methods
SEER database
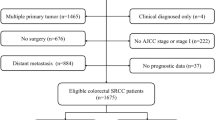
The data on all RC patients who underwent surgery between 1988 and 2011were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER database covered and published the information of cancer incidence and survival from 18 population-based cancer registries representing approximately 30% of the United States population (http://seer.cancer.gov/ about/overview.html). This version of SEER database we used had been released April 2014 (November 2013 submission). Characteristics recorded for each patient included age at diagnosis, gender, race, year of diagnosis, tumour numbers, tumour size, TNM stage, histological type, histological grade, surgery carried out and receipt of preoperative radiation therapy. Tumours were classified according to the International Classification of Diseases for Oncology (ICD-O). All TNM classification was restaged according to the criteria described in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition, 2010 (Stages I, II, III, and IV). The locally advanced RC patients (stage II and III) were included in this study. Based on the International Classification of Diseases for Oncology (third edition, ICD-O-3) coding schema, the tumour histological subtypes were identified as SRCC (8490), MC (8480, 8481) and NMC (8010, 8140–8141, 8144–8145, 8210–8211, 8220–8221, 8230–8231, 8260–8263). Histological grade was classified as well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), and undifferentiated (G4). The cancer-specific survival (CSS) time was calculated from the date of diagnosis to the date of cancer-specific death or the end of follow-up (cutoff date: December 2011). Deaths attributed to the cancer of interest (RC) were treated as events, and deaths from other causes are treated as censored observation.
Ethics Statement
This study was based on data from the SEER database, which contain no identifiers and were publicly available. We obtained permission to access research data files with the reference number 10058-Nov2013, and this study was approved by the ethics committee of Sichuan University West China Hospital. Informed consent from patients was not required due to the study’s retrospective nature. The analysis did not involve interaction with human subjects or use personal identifying information. Patient records/information was anonymized and de-identified prior to analysis, and the methods were performed in accordance with the approved guidelines.
Statistical Analysis
The R version 3.1.2 (http://www.R-project.org/) was used to perform all statistical analysis. The t test or chi-square test was used to compare clinicopathological characteristics. Survival curves were based on Kaplan–Meier method. The differences between the curves were analysed by log-rank test. Univariate and multivariate survival analyses were examined by the Cox proportional hazard models. The data were presented as hazard ratios (HR) with 95% confidence intervals (CI). All statistical tests were performed 2-sided, and P values <0.05 were considered to be statistically significant.
Additional Information
How to cite this article: Ling, C.-R. et al. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci. Rep. 7, 45334; doi: 10.1038/srep45334 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, doi: 10.3322/caac.21262 (2015).
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer 127, 2893–2917, doi: 10.1002/ijc.25516 (2010).
Hamilton, S. R. & Aaltonen, L. A. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. International Agency for Research on Cancer: Lyon, 2000 (2000).
Vallam, K. C. et al. Adenocarcinoma of the Rectum-A Composite of Three Different Subtypes With Varying Outcomes? Clinical colorectal cancer 15, e47–52, doi: 10.1016/j.clcc.2015.12.004 (2016).
Sung, C. O. et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 21, 1533–1541, doi: 10.1038/modpathol.2008.170 (2008).
Tung, S. Y., Wu, C. S. & Chen, P. C. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. The American journal of gastroenterology 91, 2195–2199 (1996).
Anthony, T., George, R., Rodriguez-Bigas, M. & Petrelli, N. J. Primary signet-ring cell carcinoma of the colon and rectum. Annals of surgical oncology 3, 344–348 (1996).
Dajani, Y. F., Zayid, I., Malatjalian, D. A. & Kamal, M. F. Colorectal cancer in Jordan and Nova Scotia: a comparative epidemiologic and histopathologic study. Cancer 46, 420–428 (1980).
Hyngstrom, J. R. et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Annals of surgical oncology 19, 2814–2821, doi: 10.1245/s10434-012-2321-7 (2012).
Bittorf, B. et al. Primary signet-ring cell carcinoma of the colorectum. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie 389, 178–183, doi: 10.1007/s00423-004-0474-y (2004).
Chew, M. H. et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. International journal of colorectal disease 25, 1221–1229, doi: 10.1007/s00384-010-1033-3 (2010).
Hugen, N., Velde, C. J. H. v. d., deWilt, J. H. W. & Nagtegaal, I. D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Annals of Oncology 25, 651–657 (2014).
Morikawa, T. et al. Prognostic significance and molecular associations of tumor growth pattern in colorectal cancer. Annals of surgical oncology 19, 1944–1953, doi: 10.1245/s10434-011-2174-5 (2012).
Han, Y. D. et al. Predictors of Pathologic Complete Response in Rectal Cancer Patients Undergoing Total Mesorectal Excision After Preoperative Chemoradiation. Medicine 94, e1971, doi: 10.1097/MD.0000000000001971 (2015).
Tomono, A. et al. Prognostic significance of pathological response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. International journal of clinical oncology 21, 344–349, doi: 10.1007/s10147-015-0900-x (2016).
Hugen, N. et al. Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Annals of surgical oncology 22, 2669–2676, doi: 10.1245/s10434-014-4339-5 (2015).
Bratland, A., Vetrhus, T., Groholt, K. K. & Ree, A. H. Preoperative radiotherapy in rectal signet-ring cell carcinoma - magnetic resonance imaging and treatment outcome: Report of six cases. Acta oncologica 49, 42–49, doi: 10.3109/02841860903081897 (2010).
E, K., CA, M. & ID, N. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345, 638–646 (2001).
Jayanand, S. B., Seshadri, R. A. & Tapkire, R. Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. International journal of colorectal disease 26, 23–27, doi: 10.1007/s00384-010-1082-7 (2011).
Lee, H. S. et al. Clinical Features and Prognosis of Resectable Primary Colorectal Signet-Ring Cell Carcinoma. Intestinal research 13, 332–338, doi: 10.5217/ir.2015.13.4.332 (2015).
Hugen, N. et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. International journal of cancer 136, 333–339, doi: 10.1002/ijc.28981 (2015).
Thota, R., Fang, X. & Subbiah, S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. Journal of gastrointestinal oncology 5, 18–24, doi: 10.3978/j.issn.2078-6891.2013.051 (2014).
Simkens, G. A. et al. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 42, 794–800, doi: 10.1016/j.ejso.2016.03.014 (2016).
Borger, M. E. et al. Signet ring cell differentiation in mucinous colorectal carcinoma. The Journal of pathology 212, 278–286, doi: 10.1002/path.2181 (2007).
Wang, F. et al. XPG rs2296147 T > C polymorphism predicted clinical outcome in colorectal cancer. Oncotarget 7, 11724–11732, doi: 10.18632/oncotarget.7352 (2016).
Lee, W. S. et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. American journal of surgery 194, 294–298, doi: 10.1016/j.amjsurg.2006.12.041 (2007).
Mekenkamp, L. J. et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. European journal of cancer 48, 501–509, doi: 10.1016/j.ejca.2011.12.004 (2012).
Colombino, M. Prevalence and prognostic role of microsatellite instability in patients with rectal carcinoma. Annals of Oncology 13, 1447–1453, doi: 10.1093/annonc/mdf240 (2002).
Negri, F. V. et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. British journal of cancer 98, 143–147, doi: 10.1038/sj.bjc.6604131 (2008).
Hongo, K. et al. Immunohistochemical detection of high-mobility group box 1 correlates with resistance of preoperative chemoradiotherapy for lower rectal cancer: a retrospective study. World journal of surgical oncology 13, 7, doi: 10.1186/1477-7819-13-7 (2015).
Yan, R. et al. Paf15 expression correlates with rectal cancer prognosis, cell proliferation and radiation response. Oncotarget, doi: 10.18632/oncotarget.9606 (2016).
Carames, C. et al. MicroRNA-21 predicts response to preoperative chemoradiotherapy in locally advanced rectal cancer. International journal of colorectal disease 30, 899–906, doi: 10.1007/s00384-015-2231-9 (2015).
Carames, C. et al. MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer. International journal of molecular sciences 17, doi: 10.3390/ijms17060878 (2016).
Svoboda, M. et al. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiation oncology 7, 195, doi: 10.1186/1748-717X-7-195 (2012).
Tsujinaka, S. et al. Long-term efficacy of preoperative radiotherapy for locally advanced low rectal cancer. International journal of colorectal disease 23, 67–76, doi: 10.1007/s00384-007-0369-9 (2008).
Chirieac, L. R. et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 11, 2229–2236, doi: 10.1158/1078-0432.ccr-04-1840 (2005).
Li, Y. et al. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. International journal of biological sciences 12, 1022–1031, doi: 10.7150/ijbs.15438 (2016).
Acknowledgements
This study was supported by grants from the National Scientific Foundation of China (Grant Nos 81401949, 81300359).
Author information
Authors and Affiliations
Contributions
C.R.L., M.J.W. and R.W. planned the research, wrote the manuscript and calculated statistics. J.P. and W.Z. supervised the entire project and helped to write the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ling, CR., Wang, R., Wang, MJ. et al. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep 7, 45334 (2017). https://doi.org/10.1038/srep45334
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45334
This article is cited by
-
The prognostic ability of radiotherapy of different colorectal cancer histological subtypes and tumor sites
Scientific Reports (2023)
-
Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the gallbladder
BMC Gastroenterology (2021)
-
Construction and validation a nomogram to predict overall survival for colorectal signet ring cell carcinoma
Scientific Reports (2021)
-
Signet ring cell component in pretreatment biopsy predicts pathological response to preoperative chemoradiotherapy in rectal cancer
International Journal of Clinical Oncology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.