Abstract
This study aimed to explore the association of tumor sidedness with the prognosis of patients with colon signet ring cell carcinoma (SRCC). Eligible patients were retrieved from the Surveillance, Epidemiology, and End Results database between 2004 and 2015. Cancer-specific survival (CSS) and overall survival (OS) were compared between patients with left-sided colon SRCC and those with right-sided lesions. A total of 2660 patients were included, among them, 1983 (74.5%) had right-sided colon SRCC. Compared to patients with left-sided colon SRCC, those who had the right-sided colon SRCC showed higher proportion of white race, female, aged ≥ 65 years, receiving total colectomy and ≥ 4 regional lymph node dissection; while had lower proportion of advanced AJCC stage. Besides, right-sided patients exhibited superior 5-year CSS (32.74% vs. 25.89%, P = 0.001) and OS (27.38% vs. 23.02%, P = 0.024) rates compared with left-sided ones. Multivariate analysis revealed that tumor sidedness was an independent prognostic factor. To be specific, patients with right-sided colon SRCC showed better CSS (HR: 0.873; 95% CI 0.777–0.981; P = 0.023) and OS (HR: 0.838; 95% CI 0.753–0.965; P = 0.002). Moreover, subgroup analysis demonstrated superior CSS and OS for right-sided patients in most subgroups. Tumor sidedness was an independent prognostic indicator for colon SRCC. Besides, patients with right-sided colon SRCC have superior prognosis than those with left-sided lesions.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) still ranks the second place among the all causes of cancer-related deaths in US, and it represents a severe health issue worldwide1. CRC can be classified intodifferent subtypes, among which, the colorectal signet ring cell carcinoma (SRCC) has aroused wide attentions recently. Laufman and Saphir first described SRCC in 19512. Primary SRCC usually occurs in stomach while rarely develops in the colorectum3,4,5,6,7,8.
In the last few years, great attention has been paid to the differentiation between left-sided and right-sided CRC. As reported in recent research, right-sided CRC shows an increasing morbidity in the last decade9,10, thus prompting us to investigate the possible causes for such variation in tumor sidedness. Recent systematic review has suggested that great heterogeneities were detected between the left-sided and right-sided CRC in terms of their pathology, epidemiology, genetic mutations and clinical presentations11. At present, no consensus is reached on the prognosis for left-sided versus right-sided CRC, and it is still controversial whether tumor location significantly affects patient prognosis12,13,14,15. Nonetheless, no study has been carried out to investigate the role of tumor sidedness on the survival for patients with colon SRCC.
The Surveillance, Epidemiology and End Results (SEER) program is supported by the National Cancer Institute (NCI). This program contains research data of 18 different population-based cancer registries, which covers 30% of the US population16. In this regard, SEER has been recognized as an efficient database for investigating such rare cancers17,18,19. This work aimed to examine the relationship of tumor sidedness with the survival of patients with colon SRCC based on the SEER database.
Materials and methods
Ethics statement
SEER data were accessed after signing the SEER Research Data Agreement (reference number, 19817-Nov2018). Data were subsequently obtained in accordance with stipulated guidelines. All data used in this study were freely accessible and did not involve human subjects (identified by the Office for Human Research Protection), thus, institution review board approval was exempted.
Study population
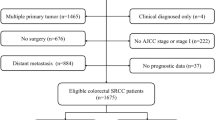
The SEER*State v8.3.6tool (August 8th, 2019) was utilized to select suitable subjects from 18 SEER regions between 2004 and 2015 (submitted in 2018). Subjects conforming to the following criteria were included: (1) patients with primary CRC; (2) pathologically confirmed SRCC according to the third edition of International Classification of Disease for Oncology (histology code: 8490/3). The exclusion criteria were listed as follows: (1) patients with multiple primary cancers; (2) clinically diagnosed alone, or based on death certificate or autopsy; (3) unavailable important information, such as surgical method and AJCC stage; (4) unknown tumor location; (5) with regard to tumor sidedness analysis (left-sided versus right-sided colon), rectal and appendiceal malignancies were eliminated; (6) without prognostic data. The remaining subjects were included as the SEER initial cohort.
Covariates and study endpoint
Patient features were analyzed based on the following factors: year of diagnosis (2004–2007, 2008–2011, 2012–2015); insured status (uninsured/unknown, any medicaid/insured), age (˂ 65, ≥ 65); marital status (unmarried, married); gender (female, male); race (black, white or others); tumor sidedness (left-sided colon, right-sided colon); grade (grade I/II, grade III/IV, unknown); tumor size (≤ 5 cm, ˃5 cm, unknown); AJCC stage (stage I, II, III, IV); surgery(no surgery, local tumor excision /partial colectomy, total colectomy), lymph node dissections (LNDs) (none or biopsy, 1–3 regional lymph nodes removed, ≥ 4 regional lymph nodes removed, unknown), chemotherapy (no/unknown, yes), radiotherapy(no/unknown, yes). Specifically, the widowed or single (never married or having a domestic partner) or divorced or separated patients were classified as unmarried. Year at diagnosis was divided into 2004–2007, 2008–2011 and 2012–2015 according to prior studies20,21. Besides, tumor sidedness was divided into left-sided colon (including splenic flexure, descending colon, sigmoid colon and rectosigmoid cancers) and right-sided colon (including cecum, ascending colon, hepatic flexure and transverse colon)12,15,22,23. Age and tumor size were grouped in accordance with prior researches24,25. In addition, the staging of cancer is based on the 6th edition of AJCC stage system, which adapted to patients in the SEER database with from 2004 to 2015.
The endpoint of this study included cancer‐specific survival (CSS) and overall survival (OS). CSS was defined as the period from diagnosis to death attributed to colon SRCC. OS was defined as the period from diagnosis to death of any cause.
Statistical analyses
Kaplan–Meier (K–M) method was employed to estimate the survival, followed by log-rank test for assessing the differences of CSS and OS. Notably, if variables had P values ≤ 0.1 in univariate analysis, they were incorporated into multivariate Cox proportional hazard analysis. Before multivariate analysis, Schoenfeld residual method was used to test the proportional risk assumption of Cox regression model, which revealed that the proportional risk assumption of this study was tenable. Similarly, Cox regression analysis was also used for stratified analysis. SPSS software (SPSS Inc., Chicago, USA, version 19.0) was used for statistical analysis, and GraphPad Prism 5 was utilized for plotting survival curves and generating forest plots. A two-sided P < 0.05 indicated statistical significance.
Results
Patient features
We identified 2660 eligible colon SRCC patients from the SEER database between 2004 and 2015, with a median follow-up of 16 months (range 0–155 months). Then, these subjects were classified into left-sided (n = 677, 25.5%) and right-sided (n = 1983, 74.5%) colon group. The detailed procedure of patient selection was presented in Fig. 1. Table 1 showed the baseline characteristics of included patients divided based on tumor sidedness. According to the results, differences in year at diagnosis (P = 0.049), insured status (P = 0.001), age (P < 0.001), gender (P < 0.001), race (P < 0.001), AJCC stage (P < 0.001), surgery (P < 0.001), LNDs (P < 0.001), chemotherapy (P < 0.001) and radiotherapy (P < 0.001) between two groups were statistically significant. Patients with right-sided colon SRCC had higher proportion of insured status (71.1% vs. 64.4%), aged ≥ 65 (53.7% vs. 36.9%), female (55.8% vs. 39.4%), white race (84.8% vs. 78.9%), receiving total colectomy (68.2% vs. 28.1%) and ≥ 4 LNDs (77.6% vs. 68.1%), while had lower proportion of advanced AJCC stage (79.2% vs. 86.5%), receiving chemotherapy (53.1% vs. 63.4%) and radiotherapy (2.2% vs. 10.0%) compared to patients with left-sided lesions.
Prognostic effect of tumor sidedness on patient
Differences in CSS (P = 0.001) and OS (P = 0.024) between right-sided and left-sided groups were statistically significant (Fig. 2). The 3-, 5- and 10-year CSS rates were 31.70%, 25.89% and 21.81% in left-sided patients, which were 39.94%, 32.74% and 28.26% in right-sided patients. Meanwhile, the 3-, 5- and 10-year OS were 28.84%, 23.02% and 15.78% for left-sided SRCC patients, which were 35.89%, 27.38% and 18.43% for right-sided patients. Univariate log-rank test identified several factors related to CSS (P ≤ 0.1), including tumor sidedness, gender, marital status, grade, tumor size, AJCC stage, surgery, LNDs, chemotherapy and radiotherapy. After adjusting for the above factors, tumor sidedness remained as an independent risk factor of CSS. Additionally, patients with right-sided colon had significantly better CSS than left-sided ones [hazard ratio (HR):0.873; 95% confidence interval (CI) 0.777–0.981; P = 0.023]. Meanwhile, all aforementioned covariates and age were significantly associated with OS. Multivariate analysis showed that patients with right-sided colon also had a favorable prognosis in terms of OS (HR: 0.838; 95% CI 0.753–0.965; P = 0.002). The detailed results of univariate and multivariate analysis were shown in Table 2.
Subgroup analysis of the effect of cancer sidedness on CSS and OS
The impacts of tumor sidedness on patient CSS and OS were determined based on subgroup analysis. As indicated by forest plots for subgroup analysis, right-sided patients had superior OS and CSS than left-sided patients in most subgroups (Figs. 3 and 4).
Discussion
To our knowledge, this is the first study to explore the effect of tumor sidedness on the survival of colon SRCC patients. In order to make our data more convincing, patients were strictly screened. First of all, only pathologically confirmed primary colon SRCC patients were selected. Then, patients with secondary primary tumor were deleted. Finally, we also deleted patients with missing important clinicopathological variables, such as AJCC stage, location, etc. Finally, a total of 2660 colon SRCC patients were enrolled into analysis. And our results indicated that right-sided patients were associated with better survival and prognosis. After adjusting for tumor and demographic features, the cancer-specific death and overall death in patients with right-sided colon SRCC were reduced by 12.7% and 16.2%, respectively, relative to those in left-sided patients. In general, tumor sidedness serves as an independent factor to predict prognosis among colon SRCC patients.
As stated in studies, right-sided CRC patients are associated with older age, female, greater tumor size, more advanced tumor stages, and poor tumor differentiation10,26,27,28. These are partly consistent with our findings, except that we found that right-sided colon SRCC tended to have earlier stage.
At present, no uniform results have been obtained for the prognosis of left-sided versus right-sided CRC, and more studies revealed poorer survival in right-sided primary tumor29,30,31. For instance, Petrelli et al. performed a systematic review and meta-analysis by recruiting 66 studies, and further suggested that left-sided CRC was correlated with the decreased mortality rate (HR: 0.82; 95% CI 0.79–0.84)29. In addition, by enrolling stage III CRC patients (n = 1,869), Taieb et al. discovered that right-sided cancer patients showed worse OS (HR: 1.25; 95% CI 1.02–1.54) compared left-sided tumor patients30. Another study revealed the association of left-sided tumor with better survival in patients with wild-type RAS metastatic CRC31.
However, different outcomes have also been suggested by other studies. By adopting propensity score matching, Rene Warschkow demonstrated that right-sided CRC patients had superior OS (HR = 0.92, 95% CI 0.8–0.94, P < 0.001) together with better CSS (HR = 0.90, 95% CI 0.87–0.93, P < 0.001)32. Generally speaking, no consensus has been reached about the prognosis for left-sided vs. right-sided CRC. In consideration of the numerous heterogeneities (such asinclusion or exclusion criteria, study design), it remains difficult to compare those available studies.
Notably, the SEER cancer registry is beneficial because it allows us to evaluate rare cancers in a great cross-section and offers the long-term follow-up data with no intrinsic institutional bias. Nonetheless, certain limitations should be noted in our study. Firstly, 2303 (46%) patients were excluded because of the strict screening criteria in this study, which may lead to certain selection biases, but it is inevitable in retrospective studies17,19. Secondly, not all required data are contained in the SEER database to comprehensively answer our questions. For instance, there is no available information regarding family history, performance status, extramural vascular invasion or obstruction/occlusion status. Thirdly, the variables of chemotherapy and radiotherapy showed low sensitivity. Only a part of patients definitely received radiotherapy or chemotherapy, which is defined as "yes"; while others are uncertain, which is defined as "no/unknown". Therefore, interpreting this part of data should be extremely careful. Finally, several important prognostic information is unavailable from the SEER database, such as Microsatellite stability/Microsatellite instability (MSS/MSI) status. Although it is better to obtain more details, we believed that the present available data from SEER database could fit our research objectives very well. The above concerns should be investigated in future studies.
Conclusions
In conclusion, our present findings suggest that tumor sidedness could serve as an independent factor to predict prognosis in patients with colon SRCC. Compared with patients with left-sided colon SRCC, the right-sided patients have superior prognosis. More in-depth research is warranted to further examine tumor heterogeneity together with the related biological factors. In addition, tumor sidedness may be identified as an independent factor when selecting the therapeutic regimen in colon SRCC management. Collaborations are valuable for merging data derived from multiple institutions in the future.
References
El-Shami, K. et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J. Clin. 65, 428–455. https://doi.org/10.3322/caac.21286 (2015).
Laufman, H. & Saphir, O. Primary linitis plastica type of carcinoma of the colon. AMA Arch. Surgery 62, 79–91. https://doi.org/10.1001/archsurg.1951.01250030082009 (1951).
Almagro, U. A. Primary signet-ring carcinoma of the colon. Cancer 52, 1453–1457. https://doi.org/10.1002/1097-0142(19831015)52:8%3c1453::aid-cncr2820520819%3e3.0.co;2-9 (1983).
Nitsche, U. et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surgery 258, 775–782. https://doi.org/10.1097/SLA.0b013e3182a69f7e (2013) ((discussion 782–773)).
Mizushima, T. et al. Primary colorectal signet-ring cell carcinoma: clinicopathological features and postoperative survival. Surg. Today 40, 234–238. https://doi.org/10.1007/s00595-009-4057-y (2010).
Hugen, N. et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int. J. Cancer 136, 333–339. https://doi.org/10.1002/ijc.28981 (2015).
Thota, R., Fang, X. & Subbiah, S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. J. Gastrointest. Oncol. 5, 18–24. https://doi.org/10.3978/j.issn.2078-6891.2013.051 (2014).
Chalya, P. L. et al. Clinicopathological patterns and challenges of management of colorectal cancer in a resource-limited setting: a Tanzanian experience. World J. Surgical Oncol. 11, 88. https://doi.org/10.1186/1477-7819-11-88 (2013).
Cucino, C., Buchner, A. M. & Sonnenberg, A. Continued rightward shift of colorectal cancer. Diseases Colon Rectum 45, 1035–1040. https://doi.org/10.1007/s10350-004-6356-0 (2002).
Saltzstein, S. L. & Behling, C. A. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J. Clin. Gastroenterol. 41, 173–177. https://doi.org/10.1097/01.mcg.0000225550.26751.6a (2007).
Hansen, I. O. & Jess, P. Possible better long-term survival in left versus right-sided colon cancer—a systematic review. Dan. Med. J. 59, A4444 (2012).
Meguid, R. A., Slidell, M. B., Wolfgang, C. L., Chang, D. C. & Ahuja, N. Is there a difference in survival between right- versus left-sided colon cancers?. Ann. Surgic. Oncol. 15, 2388–2394. https://doi.org/10.1245/s10434-008-0015-y (2008).
Suttie, S. A. et al. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis. 13, 884–889. https://doi.org/10.1111/j.1463-1318.2010.02356.x (2011).
Faivre-Finn, C. et al. Colon cancer in France: evidence for improvement in management and survival. Gut 51, 60–64. https://doi.org/10.1136/gut.51.1.60 (2002).
Weiss, J. M. et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–medicare data. J. Clin. Oncol. 29, 4401–4409. https://doi.org/10.1200/jco.2011.36.4414 (2011).
Duggan, M. A., Anderson, W. F., Altekruse, S., Penberthy, L. & Sherman, M. E. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am. J. Surgical Pathol. 40, e94–e102. https://doi.org/10.1097/pas.0000000000000749 (2016).
Dudley, R. W. et al. Pediatric choroid plexus tumors: epidemiology, treatments, and outcome analysis on 202 children from the SEER database. J. Neuro-oncol. 121, 201–207. https://doi.org/10.1007/s11060-014-1628-6 (2015).
Dudley, R. W. et al. Pediatric low-grade ganglioglioma: epidemiology, treatments, and outcome analysis on 348 children from the surveillance, epidemiology, and end results database. Neurosurgery 76, 313–319. https://doi.org/10.1227/neu.0000000000000619 (2015) ((discussion 319; quiz 319–320)).
Hankinson, T. C. et al. Short-term mortality following surgical procedures for the diagnosis of pediatric brain tumors: outcome analysis in 5533 children from SEER, 2004–2011. J. Neurosurg. Pediatrics 17, 289–297. https://doi.org/10.3171/2015.7.peds15224 (2016).
Lv, X. et al. A nomogram for predicting bowel obstruction in preoperative colorectal cancer patients with clinical characteristics. World J. Surgical Oncol. 17, 21. https://doi.org/10.1186/s12957-019-1562-3 (2019).
Wu, S. G. et al. Survival in signet ring cell carcinoma varies based on primary tumor location: a surveillance, epidemiology, and end results database analysis. Expert Rev. Gastroenterol. Hepatol. 12, 209–214. https://doi.org/10.1080/17474124.2018.1416291 (2018).
Iacopetta, B. Are there two sides to colorectal cancer?. Int. J. Cancer 101, 403–408. https://doi.org/10.1002/ijc.10635 (2002).
Lee, M. S., Menter, D. G. & Kopetz, S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. JNCCN 15, 411–419. https://doi.org/10.6004/jnccn.2017.0038 (2017).
Shi, T. et al. Chemotherapy is associated with increased survival from colorectal signet ring cell carcinoma with distant metastasis: a surveillance, epidemiology, and end results database analysis. Cancer Med. 8, 1930–1940. https://doi.org/10.1002/cam4.2054 (2019).
Wei, F. & Lyu, H. Postoperative radiotherapy improves survival in gastric signet-ring cell carcinoma: a SEER database analysis. J. Gastric Cancer 19, 393–407. https://doi.org/10.5230/jgc.2019.19.e36 (2019).
Nawa, T. et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J. Gastroenterol. Hepatol. 23, 418–423. https://doi.org/10.1111/j.1440-1746.2007.04923.x (2008).
Papagiorgis, P., Oikonomakis, I., Karapanagiotou, I., Wexner, S. D. & Nikiteas, N. The impact of tumor location on the histopathologic expression of colorectal cancer. J. BUON 11, 317–321 (2006).
Sinicrope, F. A. et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 148, 88–99. https://doi.org/10.1053/j.gastro.2014.09.041 (2015).
Petrelli, F. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 3, 211–219. https://doi.org/10.1001/jamaoncol.2016.4227 (2017).
Taieb, J. et al. Association of prognostic value of primary tumor location in stage III colon cancer with RAS and BRAF mutational status. JAMA Oncol. 4, e173695. https://doi.org/10.1001/jamaoncol.2017.3695 (2018).
Tejpar, S. et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 3, 194–201. https://doi.org/10.1001/jamaoncol.2016.3797 (2017).
Warschkow, R. et al. Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer 16, 554. https://doi.org/10.1186/s12885-016-2412-0 (2016).
Acknowledgements
We thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology, and End Results (SEER) Program.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Z.Z. Z.-w.L. conceived the study. N.Y. and S.P. searched the database and literature. D.-w.W. analyzed the data. Z.Z. drafted the manuscript. D.-w.W. revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Z., Wang, Dw., Yan, N. et al. Superior survival in right-sided versus left-sided colon signet ring cell carcinoma. Sci Rep 10, 17900 (2020). https://doi.org/10.1038/s41598-020-74926-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74926-9
This article is cited by
-
Identifying important microbial and genomic biomarkers for differentiating right- versus left-sided colorectal cancer using random forest models
BMC Cancer (2023)
-
Interactions of occult tumor spread and surgical technique on overall and disease-free survival in patients operated for stage I and II right-sided colon cancer
Journal of Cancer Research and Clinical Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.