Abstract
We report the first natural occurrence of the Fe-analogue of akimotoite, ilmenite-structured MgSiO3, a missing phase among the predicted high-pressure polymorphs of Fe-pyroxene, with the composition (Fe2+0.48Mg0.37Ca0.04Na0.04Mn2+0.03Al0.03Cr3+0.01)Σ=1.00Si1.00O3. The new mineral was approved by the International Mineralogical Association (IMA 2016-085) and named hemleyite in honour of Russell J. Hemley. It was discovered in an unmelted portion of the heavily shocked L6 Suizhou chondrite closely associated to olivine, clinoenstatite and Fe-bearing pyroxene with a composition nearly identical to that of hemleyite. We also report the first single-crystal X-ray diffraction study of a Si-bearing, ilmenite-structured phase. The fact that hemleyite formed in a meteorite exposed to high pressures (<20 GPa) and temperatures (<2000 °C) during impact-induced shocks indicates that it could play a crucial role at the bottom of the Earth’s mantle transition zone and within the uppermost lower mantle.
Similar content being viewed by others
Introduction
The mineralogy of Earth’s deep interior represents a fascinating challenge for geoscientists. Key information can be obtained from the study of mantle xenoliths1 and inclusions in diamonds2,3, as well as from experimental studies of phase equilibria of silicates and oxides4,5. However, most of the major Earth and rocky planet-forming materials [i.e., M-Si-oxides (M = Mg, Fe)] such as majorite6, akimotoite7,8, wadsleyite9, ringwoodite-ahrensite10,11, and bridgmanite7,12, have been discovered in shocked meteorites. Shocked meteorites are extraterrestrial rocks that have experienced high-pressure and high-temperature collisions in outer space, fundamental processes affecting planets and asteroids through the evolution of the solar system. As a direct product of these impact events, shock metamorphism in meteorites provides many dense silicate minerals that are thought to compose Earth’s transition zone and lower mantle. Among them, akimotoite, MgSiO3 with ilmenite structure, is thought to exist at the bottom of the Earth’s mantle transition zone and within the uppermost lower mantle, especially under low-temperature conditions13,14. Akimotoite is considered a major constituent of the harzburgite layer of subducting slabs, and the most anisotropic mineral in the mantle transition zone15,16,17. The garnet-akimotoite and akimotoite-bridgmanite phase transitions are associated with steep density and sound velocity increases and may be responsible for the multiple seismic discontinuities near the 660 km depth13,18. Due to the fact that no ilmenite or the orthorhombic perovskite modifications of FeSiO3 had been found in experiments19, most of the studies have focused on MgSiO3 akimotoite, without considering the influence of iron on its stability and density. Only recently, it was demonstrated that Fe3+-rich akimotoite is stable for a wider range of pressures than MgSiO3 akimotoite and that it may play an important role in subduction regions such as the Pacific plate beneath southern California20. Furthermore, it has been shown that Fe2+-akimotoite could derive from a progressive transformation of clinoferrosilite at pressure higher than 36 GPa21.
Here we report the discovery of the first natural occurrence of the Fe-analogue of akimotoite, with ideal formula FeSiO3. The new mineral was found in an unmelted portion of the Suizhou meteorite, and named after Russell J. Hemley (b. 1954) former Director of the Geophysical Laboratory of the Carnegie Institution of Washington D.C., USA, and very well-known in the scientific community for his research exploring the behavior of materials under extreme conditions of pressure and temperature. The new mineral and mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA 2016-085). Holotype material is deposited in the collections of the Museo di Storia Naturale, Università degli Studi di Firenze, Via La Pira 4, I-50121, Firenze, Italy, catalogue number 3238/I.
Results and Discussion
The Suizhou meteorite
This meteorite fell on April 15, 1986, in Dayanpo, which is located 12.5 km southeast of Suizhou in Hubei, China. A total of 270 kg of the Suizhou meteorite was collected, and the largest fragment, weighting 56 kg, is now preserved in the City Museum of Suizhou. This meteorite was classified as a shock-metamorphosed L6-chondrite22, with an estimated shock stage S5 according to the classification of shock metamorphism23. The meteorite contains shock-produced melt veins less than three hundred micrometers in thickness. The shock veins contains abundant high-pressure polymorphs including ringwoodite, majorite, majorite-pyrope garnet, akimotoite, magnesiowüstite, lingunite, tuite, xieite24,25,26,27,28,29,30,31,32. A (Mg,Fe)SiO3-glass was also identified in the shock veins, which was suggested to be possibly a vitrified perovskite30.
Description of the sample
The new mineral hemleyite occurs as one subhedral crystal, about 7 × 6 × 5 μm in size, coexisting with Fe-rich clinoenstatite (which is mineralogically a clinoferrosilite but we will refer at it as Fe-rich clinoenstatite or Fe-pyroxene) and closely associated to Fe-poor clinoenstatite and forsteritic olivine (Fig. 1). The chemical composition of these phases is given in Table 1. Color, lustre, streak, hardness, tenacity, cleavage, fracture, density, and optical properties could not be determined because of the small grain size. The calculated density using the empirical formula and X-ray single-crystal data is 4.383 g·cm−3.
Hemleyite was initially identified by Raman spectroscopy. The Raman spectrum of hemleyite (Fig. 2) displays bands at 795, 673, 611, 476, 403 and 342 cm−1 with the typical strong peak at 795 cm−1, corresponding to the stretching vibrations of the SiO6 octahedra. The hemleyite Raman spectrum is similar to that obtained for akimotoite from the Suizhou meteorite32 and those for akimotoite from other L-group chondrites33,34,35,36. As already observed for akimotoite from Suizhou32, Raman bands of hemleyite are much sharper than those observed for other ilmenite-type polymorphs in other chondrites, thus indicating rather high crystallinity.
To get more information on the crystal structure and the relationships with the surrounding minerals, the small hemleyite fragment was handpicked from the polished section under a reflected light microscope from the upper part of the grain in Fig. 1B and mounted on a 5 μm diameter carbon fiber, which was, in turn, attached to a glass rod. Then, the fragment was tested by single-crystal X-ray diffraction and was found to consist of crystalline hemleyite (single-crystal spots in Figure S1) associated to minor, fine-grained polycrystalline pyroxene (diffraction rings in Figure S1).
Crystal structure of hemleyite
The crystal structure of hemleyite is shown in Fig. 3. It consists of a lattice of hexagonal close-packed O atoms in which only two-thirds of the octahedral sites are occupied. The octahedra share edges to form 6-membered rings, thus forming sheets parallel to (0001). The sheets are linked into a framework by sharing faces and corners of octahedra. In hemleyite, the presence of (Fe,Mg) and Si specifically ordered into two octahedral sites (i.e., A and B) causes a decrease in symmetry from the corundum-type structure, space group R-3c, to R-3. Final atomic coordinates and equivalent isotropic displacement parameters are given in Table S1, anisotropic displacement parameters in Table S2, whereas selected bond distances are shown in Table S3.
The A site showed a mean electron number of 18.7 and the B site 14.0. The latter was thought to be fully occupied by silicon. The site population inferred at the larger A octahedral position (Fe2+0.48Mg0.37Ca0.04Na0.04Mn2+0.03Al0.03Cr3+0.01) gives a mean electron number of 19.6, in good agreement with the site scattering observed at this site. Moreover, taking into account the weighted sum of ionic radii37, we get a mean ideal bond value of 2.14 Å, close to the observed value from the structure refinement (<A-O> = 2.13 Å; Table S3). These crystal-chemical considerations, together with the perfect charge balance of the formula, point to the presence of Fe (and Mn) and Cr in the divalent and trivalent states, respectively.
The unit-cell parameters of hemleyite are strongly influenced by the entry of Fe into the structure. We observed a general expansion of the unit cell from pure MgSiO338. The assignment of Fe substituting for Mg at the A site is required both to account for the electron density at that site and to justify the increase of the mean bond distance (2.13 Å) relative to pure MgSiO3 (2.077 Å; Horiuchi et al.38). Similar to what happens in other Mg-Fe silicates39, the Fe–for–Mg substitution does not induce a distortion of the A-octahedral site (σ2 = 140.3 and 143.4 in hemleyite and pure MgSiO3, respectively). On the other hand, the Fe–for–Mg substitution has effects on the neighbouring Si-bearing B-octahedral site, with an increase of σ2 (Robinson et al.40) from 52.8 in pure MgSiO338 to 63.9 in hemleyite. If we calculate the difference between the O–O edge of the A and B sites, which is directed along the c-axis, and plot it as a function of the ratio between the ionic radius of the B and A cations (Fig. 4), an almost linear trend can be observed, with hemleyite falling close to ideal FeSiO341.
The difference between the O-O edge of the A and B sites (in Angstrom), which is directed along the c-axis, plotted as a function of the ratio between the ionic radius of the B and A cation. Filled circle corresponds to hemleyite (this study), whereas empty circles refer to literature data as follows: FeSiO341, MgSiO338, MnTiO359, FeTiO360, MgTiO361.
Crystal-chemical considerations
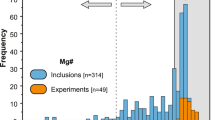
If we plot the Fe and Mg contents of all the akimotoites reported in literature8,32,33,35,42,43,44,45,46,47,48,49, we can observe that a discrete variation is present, with only the phase from the Suizhou meteorite described here crossing the boundary delimiting akimotoite and hemleyite (dashed line in Fig. 5). Although the chemical composition of hemleyite is far from the ideal FeSiO3 end member, Fe is the dominant cation at the A site of the ilmenite-type structure. Furthermore, we always found Fe > Mg in all the microprobe analyses carried out on the grain depicted in Fig. 1B (Table 1) and used for the structural investigation.
Another interesting feature is related to the minor (Ca + Na) amounts in the ilmenite structure. Minor contents of these elements were detected in all the reported akimotoite occurrences8,32,33,35,42,43,44,45,46,47,48,49. With increasing the Fe/(Fe + Mg) ratio, an increase of the (Ca + Na) content in akimotoite is evident (Fig. 6). The only value going off the main trend is the akimotoite from the Tenham meteorite studied by Ferroir et al.35. The presence of Fe2+replacing Mg induces an increase of the size of the A octahedra and, more generally, an overall enlargement of the ilmenite-structure topology. Such an enlargement favors the entry of larger cations, such as Ca and Na.
Formation hypothesis for hemleyite
Static high-pressure experiments demonstrated that low-Ca pyroxene could transform to akimotoite at 17.5–27.5 GPa and 600–2050 °C19,50. The transformation of enstatite to akimotoite requires temperatures in excess of 1550 °C at 22 GPa51. Two scenarios or models were proposed for the formation mechanisms of akimotoite in shock-metamorphosed meteorites: (1) solid state transformation from low-Ca pyroxene or clinopyroxene to akimotoite under high pressures and temperatures7,8; (2) crystallization from a chondritic silicate melt under high pressures and temperature42. The first model is mainly based on a topotaxial relationship between akimotoite with neighboring pyroxene, and the same composition between pyroxene and akimotoite7,8, and the second one is because akimotoite is enriched in CaO, Al2O3 and Na2O with respect to the coexisting pyroxene42. In the Suizhou L6 chondrite, hemleyite occurs mainly along the cracks within silicates (Mg-rich pyroxene and Mg-rich olivine). The hemleyite fragment we have studied (Fig. 1B) was found to intimately coexist with Fe-rich clinoenstatite and be surrounded by Fe-poor clinoenstatite. Such petrographic evidence could lead to the following formation mechanism: (1) Fe-rich clinoenstatite belongs to a kind of secondary pyroxene likely formed from olivine and pyroxene by replacement of Fe-rich materials during the thermal metamorphism of the meteorite, and (2) a later impact-induced shock transformed some Fe-rich clinoenstatite into hemleyite. The pressure and temperature conditions (18–20 GPa and 1800–2000 °C) of shock-veins in the Suizhou meteorite32 can be estimated according to the liquidus phases of high-pressure minerals. However, the P–T conditions for the formation of hemleyite in the chondritic portion cannot be estimated because of the lack of available phase diagram for the solid-state crystallization of ilmenite-structured (Fe,Mg)SiO3.
Implications
Neither Fe-dominant MgSiO3 nor pure FeSiO3 with ilmenite structure have been synthesized yet. Indeed, high-pressure experiments52 in the system MgSiO3-FeSiO3 at about 23 GPa at 1100 °C show that ilmenite cannot contain more than 15 mol% of FeSiO3. For FeSiO3-richer compositions, the experimental products are ilmenite s.s. (FeSiO3) + ringwoodite s.s (Mg2SiO4) + stishovite (SiO2). We think that the difference between the experimental results52 and what shown in the present study is not linked to the extremely rapid cooling rates occurring in impact-induced shocks, which can allow to discover phases that would not be experimentally recovered under ambient conditions. The most likely reasons could be (i) a metastable transition of Fe-rich pyroxene to hemleyite, or (ii) a temperature-dependent increase of solubility of the FeSiO3 component in the ilmenite structure. The first hypothesis is that inferred to explain the transition of Na-rich hollandite (lingunite) from feldspar53. The second, which seems more unlikely, cannot be properly weighted at present as experimental data are still insufficient to evaluate how much FeSiO3 component is dissolved in ilmenite in the range of temperature 1800–2000 °C.
The discovery of the Fe-dominant akimotoite, i.e. hemleyite, is important not only to shed further light on asteroidal impact events but also for mantle geophysics as it expands the knowledge of the most important mantle minerals. To know that also ilmenite-structured MgSiO3 can contain high Fe contents is important to study the effect of this chemical substitution on the acoustic velocity and density profiles in high-pressure models and for a comparison with seismically derived Earth velocity and density structure obtained with computational approaches54. The ideas of multiple seismic discontinuities near the 660 km boundary in the subduction zone55, instead of a single, sharp velocity jump at the lower-mantle boundary, strongly suggest the presence of akimotoite/hemleyite solid solution at this depth in the peridotitic lithology of subducted slabs.
Methods
Studied material
A small piece of the Suizhou meteorite (about 10 g), was sliced in 6 different pieces. Each piece was embedded in epoxy, polished with diamond pastes, and then studied by scanning electron microscopy and electron microprobe.
Scanning electron microscopy
The instrument used was a Zeiss - EVO MA15 Scanning Electron Microscope coupled with an Oxford INCA250 energy-dispersive spectrometer, operating at 25 kV accelerating potential, 500 pA probe current, 2,500 cps as average count rate on the whole spectrum, and a counting time of 500 s. Samples were sputter-coated with 30-nm-thick carbon film.
Electron microprobe
Quantitative analyses on the different phases were carried out using a JEOL JXA 8600 microprobe (WDS mode, 15 kV, 10 nA, 1 μm beam size, counting times 20 s for peak and 10 s for background). High spatial resolution was achieved using conditions of 13 kV, 7 nA. For the WDS analyses the Kα lines for all the elements were used. The standards employed were: albite (Na, Al, Si), synthetic Cr2O3 (Cr), ilmenite (Fe), olivine (Mg), diopside (Ca), and bustamite (Mn).
Single-crystal X-ray diffraction and structure refinement
Single-crystal X-ray studies were carried out using a Oxford Diffraction Xcalibur 3 diffractometer equipped with an Oxford Diffraction CCD detector, with graphite-monochromatized MoKα radiation (λ = 0.71073 Å), working conditions 50 kV × 50 nA and with 300 s exposure time per frame; the detector-to-sample distance was 6 cm. Hemleyite is trigonal, space group R-3, with unit-cell parameters (hexagonal setting): a = 4.7483(5), c = 13.665(1) Å, V = 266.82(6) Å3, c:a = 2.8779, Z = 6.
Single-crystal X-ray diffraction intensity data of hemleyite were integrated and corrected for standard Lorentz and polarization factors with the CrysAlis RED package56. The program ABSPACK in CrysAlis RED56 was used for the absorption correction. A total of 270 unique reflections was collected. Given the similarity in the unit-cell values and in the space groups, the structure was refined starting from the atomic coordinates reported for akimotoite37 using the program Shelxl-9757. The site occupation factor (s.o.f.) at the cation sites was allowed to vary (Fe vs. Mg and Si vs. structural vacancy for the A and B sites, respectively) using scattering curves for neutral atoms taken from the International Tables for Crystallography58. In terms of mean electron number, the X-ray formula, (Fe0.48Mg0.52)SiO3, is in excellent agreement with that obtained with the electron microprobe, (Fe2+0.48Mg0.37Ca0.04Na0.04Mn2+0.03Al0.03Cr3+0.01)Σ=1.00Si1.00O3. At the last refinement stage, with anisotropic atomic displacement parameters for all atoms and no constraints, the residual value settled at R1(F) = 0.0593 for 187 observed reflections [Fo > 4σ(Fo)] and 17 parameters and at R1(F) = 0.0892 for all 270 independent reflections.
Crystallographic data (CCDC 1522131) can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
X-ray powder diffraction
X-ray powder diffraction data (Table S4) were obtained with an Oxford Diffraction Xcalibur PX Ultra diffractometer fitted with a 165 mm diagonal Onyx CCD detector and using copper radiation (CuKα, λ = 1.54138 Å). The working conditions were 50 kV × 50 nA with 7 hours of exposure; the detector-to-sample distance was 7 cm. The program Crysalis RED56 was used to convert the observed diffraction rings to a conventional powder diffraction pattern. The least squares refinement gave the following unit-cell values: a = 4.7490(2), c = 13.6934(9) Å, V = 267.45(2) Å3.
Additional Information
How to cite this article: Bindi, L. et al. Discovery of the Fe-analogue of akimotoite in the shocked Suizhou L6 chondrite. Sci. Rep. 7, 42674; doi: 10.1038/srep42674 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Collerson, K. D., Hapugoda, S., Kamber, B. S. & Williams, Q. Rocks from the mantle transition zone: Majorite-bearing xenoliths from Malaita, Southwest Pacific. Science 288, 1215–1223 (2000).
Moore, R. O. & Gurney, J. J. Pyroxene solid solution in garnets included in diamond. Nature 318, 553–555 (1985).
Walter, M. J. et al. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 334, 54–57 (2011).
Gasparik, T. Phase Diagrams for Geoscientists. Springer-Verlag, Berlin (2003).
Irifune, T. & Tsuchiya, T. In Treatise on Geophysics, G. D. Price, Ed. vol. 2, 33–62 (2007) (Elsevier, Amsterdam, 2007).
Tomioka, N., Miyahara, M. & Ito, M. Discovery of natural MgSiO3 tetragonal garnet in a shocked chondritic meteorite. Sci. Adv. 2, e1501725 (2016).
Tomioka, N. & Fujino, K. Natural (Mg,Fe)SiO3-ilmenite and -perovskite in the Tenham meteorite. Science 277, 1084–1086 (1997).
Tomioka, N. & Fujino, K. Akimotoite, (Mg,Fe)SiO3, a new silicate mineral of the ilmenite group in the Tenham chondrite. Am. Mineral. 84, 267–271 (1999).
Price, R. D., Putnis, A., Agrell, S. O. & Smith, D. G. W. Wadsleyite, natural beta-(Mg,Fe)2SiO4 from the Peace River meteorite. Can. Mineral. 21, 29–35 (1983).
Binns, R. A., Davis, R. J. & Reed, S. J. B. Ringwoodite, natural (Mg,Fe)2SiO4 spinel in the Tenham meteorite. Nature 221, 943–944 (1969).
Ma, C. et al. Ahrensite, γ-Fe2SiO4, a new shock-metamorphic mineral from the Tissint meteorite: Implications for the Tissint shock event on Mars. Geoch. Cosmoch. Acta 184, 240–256 (2016).
Tschauner, O. et al. Discovery of bridgmanite, the most abundant mineral in Earth, in a shocked meteorite. Science 346, 1110–1112 (2014).
Akaogi, M., Tanaka, A. & Ito, E. Garnet–ilmenite–perovskite transitions in the system Mg4Si4O12–Mg3Al2Si3O12 at high-pressures and high-temperatures: phase equilibria, calorimetry and implications for mantle structure. Phys. Earth Planet. Inter. 132, 303–324 (2002).
Shiraishi, R. et al. Crystallographic preferred orientation of akimotoite and seismic anisotropy of Tonga slab. Nature 455, 657–660 (2008).
Weidner, D. J. & Ito, E. Elasticity of MgSiO3 in the ilmenite phase. Phys. Earth Planet. Inter. 40, 65–70 (1985).
Da Silva, C. R. S., Karki, B. B., Stixrude, L. & Wentzcovitch, R. M. Ab initio study of the elastic behavior of MgSiO3 ilmenite at high-pressure. Geophys. Res. Lett. 26, 943–946 (1999).
Zhang, Y., Zhao, D. & Matsui, M. Anisotropy of akimotoite: A molecular dynamics study. Phys. Earth Planet. Inter. 151, 309–319 (2005).
Wang, Y. et al. Thermal equation of state of akimotoite MgSiO3 and effects of the akimotoite–garnet transformation on seismic structure near the 660 km discontinuity. Phys. Earth Planet. Int. 143–144, 57–80 (2004).
Liu, L.-G. The high-pressure phases of FeSiO3 with implications for Fe2SiO4 and FeO. Earth Plan. Sci. Lett. 33, 101–106 (1976).
Liu, J. et al. The stability and density of Fe-bearing akimotoite and implications for subduction. Am. Geoph. Union Fall Meet. 2016, Abstract#MR33A-2680 (2016).
Pakhomova, A. et al. A new high pressure phase transition in clinoferrosilite: in situ single crystal X-ray diffraction study. Am. Mineral. 10.2138/am-2017-5853 (2017).
Wang, R. & Li, Z. Comprehensive studies on the Shuizhou meteorite. Publishing House of the China University of Geosciences, Wuhan (1990).
Stöffler, D., Keil, K. & Scott, E. R. D. Shock metamorphism of ordinary chondrites. Geoch. Cosmoch. Acta 55, 3845–3867 (1991).
Xie, X. D., Chen, M. & Wang, D. Q. Shock-related mineralogical features and P-T history of the Suizhou L6 chondrite. Eur. J. Mineral. 13, 1177–1190 (2001).
Xie, X. D. et al. Tuite, γ-Ca3(PO4)2, a new phosphate mineral from the Suizhou L6 chondrite. Eur. J. Mineral. 15, 1001–1005 (2003).
Xie, X. D., Chen, M. & Wang, D. Q. Two types of silicate melts in naturally shocked meteorites. In: Papers and abstracts of the 5th Annual Meeting of IPACES, Guangzhou. 12–14 (2005).
Xie, X. D., Sun, Z. Y. & Chen M. The distinct morphological and petrological features of shock melt veins in the Suizhou L6 chondrite. Met. Plan. Sci. 46, 459–469 (2011).
Chen, M., Shu, J. F., Xie, X. D. & Mao, H.-K. Natural CaTi2O4-structured FeCr2O4 polymorph in the Suizhou meteorite and its significance in mantle mineralogy. Geoch. Cosmoch. Acta 67, 3937–3942 (2003).
Chen, M. et al. Natural occurrence and synthesis of two new postspinel polymorphs of chromite. Proc. Nat. Acad. Sci. USA 100, 14651–14654 (2003).
Chen, M., Xie, X. D. & El Goresy, A. A shock-produced (Mg,Fe)SiO3 glass in the Suizhou meteorite. Met. Plan. Sci. 39, 1797–1808 (2004).
Chen, M., Shu, J. F. & Mao, H.-K. Xieite, a new mineral of high-pressure FeCr2O4 polymorph. Chinese Sci. Bull. 53, 3341–3345 (2008).
Chen, M. & Xie X. D. Shock-produced akimotoite in the Suizhou L6 chondrite. Sci. China Earth Sci. 58, 876–880 (2015).
Ohtani, E. et al. Formation of high-pressure minerals in shocked L6 chondrite Yamato 791384: Constraints on shock conditions and parent body size. Earth Plan. Sci. Lett. 227, 505–515 (2004).
Zhang A. C. Pyroxene polymorphs in melt veins of the heavily shocked Sixiangkou L6 chondrite. Eur. J. Mineral. 18, 719–726 (2006).
Ferroir, T. et al. Akimotoite in the Tenham meteorite: Crystal chemistry and high-pressure transformation mechanisms. Earth Plan. Sci. Lett. 275, 26–31 (2008).
Nagy, Sz. et al. Olivine and pyroxene high-pressure polymorphs in melt veins of the strongly shocked NWA 5011 meteorite sample. In: 41st Lunar Planetary Science Conference, Houston, No.1228 (2010).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A32, 751–767 (1976).
Horiuchi, H., Hirano, M., Ito, E. & Matsui, Y. MgSiO3 (ilmenite-type): single crystal X-ray diffraction study. Am. Mineral. 67, 788–793 (1982).
Griffen, D. T. Silicate Crystal Chemistry. New York and Oxford (Oxford University Press), 442 pp (1992).
Robinson, K., Gibbs, G. V. & Ribbe, P. H. Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172, 567–570 (1971).
Ribeiro, R. A. P. & de Lázaro, S. R. Structural, electronic and elastic properties of FeBO3 (B = Ti, Sn, Si, Zr) ilmenite: a density functional theory study. RSC Adv. 4, 59839 (2014).
Sharp, T. G., Lingemann, C. M., Dupas, C. & Stöffler, D. Natural occurrence of MgSiO3-ilmenite and evidence for MgSiO3-perovskite in a shocked L chondrite. Science 277, 352–255 (1997).
Langenhorst, F. & Poirier, J.-P. Anatomy of black veins in Zagami: clues to the formation of high-pressure phases. Earth Plan. Sci. Lett. 184, 37–55 (2000).
Xie, Z. & Sharp, T. G. High-pressure phases in shock-induced melt veins of the Umbarger L6 chondrite: Constraints of shock pressure. Met. Plan. Sci. 3912, 2043–2054 (2004).
Xie, Z., Sharp, T. G. & DeCarli, P. S. High-pressure phases in a shock-induced melt vein of the Tenham L6 chondrite: Constraints on shock pressure and duration. Geoch. Cosmoch. Acta 70, 504–515 (2006).
Zhang, A., Hsu, W., Wang, R. & Ding, M. Pyroxene polymorphs in melt veins of the heavily shocked Sixiangkou L6 chondrite. Eur. J. Mineral. 18, 719–726 (2006).
Ozawa, S. et al. Transformation textures, mechanisms of formation of high-pressure minerals in shock melt veins of L6 chondrites, and pressure-temperature conditions of the shock events. Met. Plan. Sci. 4411, 1771–1786 (2009).
Miyajima, N. et al. Ferric iron in Al-bearing akimotoite coexisting with iron-nickel metal in a shock-melt vein in an L-6 chondrite. Am. Mineral. 92, 1545–1549 (2007).
Imae, N. & Ikeda, Y. High-pressure polymorphs of magnesian orthopyroxene from a shock vein in the Yamato-000047 lherzolitic shergottite. Met. Plan. Sci. 451, 43–54 (2010).
Presnell, D. C. Phase diagrams of earth-forming minerals. In: Ahrens T. J., ed. Mineral Physics and Crystallography: A Handbook of Physical Constants. American Geophysical Union. 248–268 (1995).
Hogrefe, A., Rubie, D. C., Sharp, T. G. & Seifert, F. Metastability of enstatite in deep subducting lithosphere. Nature 372, 351–353 (1994).
Ito, E. & Yamada, H. Stability relations of silicate spinels, ilmenites, and perovskites. In S. Akimoto & M. H. Manghnani, Eds, High-Pressure Research in Geophysics, p. 405–419. Center of Academic Publications, Tokyo (1982).
Tomioka, N., Mori, H. & Fujino, K. Shock induced transition of NaAlSi3O8 feldspar into a hollandite structure in a L6 chondrite. Geophys. Res. Lett. 27, 3997–4000 (2000).
Weidner, D. J. & Wang, Y. Chemical and Clapeyron induced buoyancy at the 660 km discontinuity. J. Geophys. Res. 103, 7431–7442 (1998).
Niu, F. & Kawakatsu, H. Complex structure of mantle discontinuities at the tip of the subducting slab beneath northeast China. J. Phys. Earth 44, 701–711 (1996).
Oxford Diffraction. CrysAlis RED (Version 1.171.31.2) and ABSPACK in CrysAlis RED. Oxford Diffraction Ltd, Abingdon, Oxfordshire, England (2006).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A64, 112–122 (2008).
Wilson, A. J. C., Ed. International Tables for Crystallography, Volume C: Mathematical, physical and chemical tables. Kluwer Academic, Dordrecht, NL (1992).
Kidoh, K., Tanaka, K., Marumo, F. & Takei, H. Electron density distribution in ilmenite-type crystals. II. Manganese (II) titanium (IV) trioxide. Acta Crystallogr. B40, 329–332 (1984).
Yamanaka, T., Komatsu, Y. & Nomori, H. Electron density distribution of FeTiO3 ilmenite under high pressure analyzed by MEM using single crystal diffraction intensities. Phys. Chem. Min. 34, 307–318 (2007).
Wechsler, B. A. & Von Dreele, R. B. Structure refinements of Mg2TiO4, MgTiO3 and MgTi2O5 by time-of-flight neutron powder diffraction. Acta Crystallogr. B45, 542–549 (1989).
Acknowledgements
This work was supported by “Progetto di Ateneo 2015” of the University of Florence, Italy (L.B.) and the National Natural Science Foundation of China under Grant No. 41672032 (M.C.) and No. 41172046 (X.X.). SEM studies were conducted at the MEMA laboratory of the University of Florence, Italy. EPMA analyses were carried out at the Filippo Olmi laboratory of the University of Florence, Italy. Single-crystal and powder X-ray diffraction studies were done at CRIST, Centro di Cristallografia Strutturale, University of Florence, Italy.
Author information
Authors and Affiliations
Contributions
The study was conceived and guided by L.B., who also performed the SEM, electron microprobe and X-ray diffraction studies. L.B. wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bindi, L., Chen, M. & Xie, X. Discovery of the Fe-analogue of akimotoite in the shocked Suizhou L6 chondrite. Sci Rep 7, 42674 (2017). https://doi.org/10.1038/srep42674
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42674
This article is cited by
-
The breakdown of diopside to (Ca, Mg)SiO3 perovskite–(Mg, Ca, Fe)SiO3 glass–(Mg, Ca)SiO3 glass–(Mg, Ca)SiO3 majorite in a melt vein the Suizhou L6 chondrite
Acta Geochimica (2023)
-
The discovery of TiO2-II, the α-PbO2-structured high-pressure polymorph of rutile, in the Suizhou L6 chondrite
Acta Geochimica (2023)
-
Can quasicrystals survive in planetary collisions?
Progress in Earth and Planetary Science (2021)
-
Natural and experimental high-pressure, shock-produced terrestrial and extraterrestrial materials
Progress in Earth and Planetary Science (2021)
-
Poirierite, a dense metastable polymorph of magnesium iron silicate in shocked meteorites
Communications Earth & Environment (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.