Abstract
Vessel wall stiffening is an important clinical parameter, but it is unknown whether platelets, key elements in the pathogenesis of arterial thrombosis, are associated with arterial stiffness. The present studies sought to determine whether mean platelet volume (MPV), a potential marker of platelet activation, is linked to vascular elasticity as assessed by the augmentation index (AIx), in 15,010 individuals from the population-based Gutenberg Health Study. Multivariable analysis showed that MPV in both males (β 0.776; 95thCI [0.250;1.16]; p = 0.0024) and females (β 0.881[0.328;1.43]; p = 0.0018) is strongly associated with AIx. Individuals with MPV and AIx above the sex-specific medians had worse survival. Association analysis between MPV-related genetic variants and arterial stiffness identified four genetic variants in males and one in females related with AIx. Cox regression analysis for mortality identified one of these joint genetic variants close to ring finger protein 145 gene (RNF145, rs10076782) linked with increased mortality (hazard ratio 2.02; 95thCI [1.35;3.02]; p = 0.00061). Thus, these population-based data demonstrate a close relation between platelet volume as a potential marker of platelet activation and arterial stiffness in both sexes. Further research is warranted to further elucidate the mechanisms underlying larger platelets‘ role in arterial stiffening including the role of shared common genetics.
Similar content being viewed by others
Introduction
Considering the high morbidity and mortality burden triggered by atherosclerosis, efforts are clearly directed towards early recognition and diagnosis of subclinical atherosclerosis. Arterial stiffness as a measure of distensibility and compliance of vessels is an established marker of cardiovascular morbidity and mortality and therefore a potential therapeutic target1,2. Arterial hypertension and aging are established factors contributing to arterial stiffness and the role of inflammation in decreasing vascular compliance is becoming more evident as well3,4.
The role of platelets in causing increased vascular stiffness is much less well defined5,6,7,8,9. Activated platelets release a numerous number of substances that control vascular permeability, regulate vasoconstriction or vasodilatation, stimulate macrophage recruitment and infiltration, release reactive oxygen species (ROS) and stimulate ROS generation within the vascular wall leading to decreased vascular nitric oxide (NO) bioavailability10. Therefore, it is of outmost importance to better understand the interaction between arterial stiffness and parameters of platelet activation such as mean platelet volume (MPV) with regard to underlying cellular, molecular and genetic mechanisms and their clinical impact.
Several studies suggest that vessel wall stiffening is an important clinical target, but it is unknown to what extent platelets, key elements in the pathophysiology of arterial thrombosis, are associated with arterial stiffness. The Gutenberg Health Study (GHS), as a large, prospective population-based cohort study in Western Germany, offers the potential to explore this relation in depth. To the best of our knowledge, this is the first study to investigate the relation between mean platelet volume (MPV), a potential marker of platelet activation including its known genetic variants, and vascular stiffness, in a large adult general population study sample.
Methods
Population sample
Arterial stiffness and MPV have been determined in 15,010 participants enrolled in the GHS at a baseline examination in the period between April 2007 and April 2012. GHS is a population-based, prospective, observational, single-center cohort study in Western Mid-Germany designed predominantly for examination of cardiovascular disease. Residents of the City of Mainz and the County Mainz-Bingen aged 35 to 74 were selected randomly from the local governmental registry offices with a stratification for sex, residence (urban vs. rural), and decade of age. All participants underwent a 5-hour examination at the study center following standard operating procedures (SOPs) by certified medical technical assistants. Details of the study design have been published earlier11. The study protocol was approved by the Ethics Committee of the State Chamber of Physicians of Rhineland-Palatinate, Germany (Ref.No. 837.020.07(5555)) and the study project was approved by the steering committee of the Gutenberg Health Study. The study has been conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before entering the study.
Assessment of arterial stiffness
Arterial stiffness was assessed by digital volume plethysmography using an EndoPat 2000 device (Itamar Medical, Caesarea, Israel), which is based on the fingertip measurement of pulsatile volume changes. The resultant augmentation index (AIx) is automatically calculated by identifying the early (P1) and late systolic peak (P2) with the following formula: (P2-P1)/P1*100. Endo-PAT 2000 has been already validated in many studies for both arterial stiffness and endothelial function measurements12,13,14,15,16.
Blood sampling and laboratory measurements
Venous blood sampling was performed using tubes containing K3-ethylenediaminetetraacetic acid (EDTA). Platelet parameters (count and MPV) have been automatically determined on an Advia 120 Hematology System (Siemens, Germany). Coefficients of variation for MPV range between 0.8 and 2.2% over time with a mean of 1.4%. Details of internal quality control for Advia 120 hematology systems and validation for inter-instrument variation for MPV measurements have been published earlier17. Biochemical analysis of blood glucose and C-reactive protein measured in plasma and total cholesterol, high density lipoprotein (HDL), and triglycerides measured in serum were determined within 1 hour after sampling by routine methods. Low density lipoprotein (LDL) cholesterol was calculated by Friedewald´s formula. All laboratory measurements have been performed in the central laboratory of University Medical Center Mainz.
Genotyping and imputation of Single Nucleotide Polymorphisms (SNPs)
From the genetic data available for study participants, we systematically searched and selected 51 SNPs (see Supplemental Table 1) which had been described by large genome wide association studies (GWAS) for MPV to test them for association with arterial stiffness18,19,20,21. For the available genetic information, genotyping was conducted on the Affymetrix Genome-Wide Human SNP 6.0 array (Affymetrix, Santa Clara, CA) according to the manufacturer´s recommendations. After quality control, genetic data were available for analysis in 4,175 individuals. The methodology for genotyping has been described before17.
Statistical analysis
Variable of arterial stiffness derived from digital plethysmography was Augmentation Index (AIx) expressed as percentage augmentation (%). Distribution of MPV is presented as median with interquartile range and tested with the Mann–Whitney U test. Normally distributed continuous variables are described using mean ± standard deviation (SD) and tested with T-test, whereas categorical variables are displayed by absolute and relative frequency and tested by Chi-squared test if needed. The reference group is a subsample of the population sample and included 2,660 individuals without manifest disease (i.e. myocardial infarction, coronary artery disease, stroke, peripheral arterial disease, venous thromboembolism, atrial fibrillation, chronic heart failure, cancer, kidney disease, liver disease, chronic obstructive pulmonary disease and anemia) and no known traditional cardiovascular risk factors (i.e. hypertension, obesity, dyslipidemia, diabetes mellitus, smoking and positive family history (FH) for myocardial infarction (MI) or stroke). Relations between continuous variables were explored by Pearson´s correlation coefficients. Multivariable linear regression analysis with AI as dependent variable and adjustment for age, cardiovascular risk factors (CVRFs), comorbidities and medication was used to identify determinants of arterial stiffness. The analysis of prospective mortality data with censoring is presented by using plots of Kaplan Meier curves with log-rank test and by Cox proportional hazards models.
Because of the explorative character of the analysis, a significance threshold was not defined for p-values. P-values should be interpreted as a continuous measure of statistical evidence.
Results
Arterial stiffness and MPV
Arterial stiffness in the population sample without traditional CVRFs and in subsamples with CVRFs is presented sex-specifically in Fig. 1. Presented AIx values are estimated for the subgroups below the 5th% and above 95th% of MPV. In general, individuals with CVRFs had higher AIx compared to the sample without CVRFs. Males with a CVRF had higher AIx. Differently, in females, only those with hypertension, smoking, dyslipidemia and positive FH for MI or stroke had higher AIx with respect to the sample without CVRFs.
As shown in Table 1, AIx increased with increasing MPV quartiles in both males and females. When stratifying the population sample for sex-specific medians of MPV (males: 8.4 fL; females 8.5 fL) and AIx (males: 8.90%; females: 20.6%) as cut-off values, approx. 25% of the population sample had AIx and MPV with both parameters above the respective median value (Fig. 2). Within individuals with CVRFs, most subgroups had 30% and more of individuals with these characteristics. The highest proportion was observed for individuals with diabetes mellitus with 35% (95% CI: 32%; 38%) having AIx and MPV above the medians.
The unadjusted and the multivariable linear regression model for arterial stiffness, with adjustment for age, CVRFs, comorbidities, medication, fibrinogen and hematocrit confirmed the association between higher MPV and AIx for both males and females (Table 2). Adjusting the model of AIx in females additionally for the hormonal status (i.e. intake of oral contraceptives, hormone replacement therapy and presence of menstrual bleeding) did not change the observed association between MPV and AIx (β estimate: 0.968 [0.39; 1.54], p = 0.00096). Differently, as shown in the Table 2, platelet count was weakly associated to AIx for both sexes in a crude analysis and the association was lost in the multivariable adjusted models.
Association between MPV related SNPs and arterial stiffness
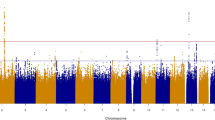
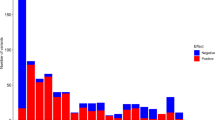
Linear regression models for the associations between MPV related SNPs and AIx in males are presented in Table 3. Of the 51 SNPs associated with MPV, 4 SNPs showed an association with AIx in males. Three SNPs showed a positive association (rs7342306, rs10076782 and rs17397129) and 1 SNP (rs7961894) a negative association with AIx. After adjusting for age and CVRFs the association remained for rs17397129 (β estimate: 1.60 [0.114; 3.08], p = 0.035). At the next step, further adjusting for comorbidities and medication no association remained significant. In females, rs11734132 was inversely associated to AIx both in the crude and adjusted analysis for age and CVRFs (β estimate: −1.92 [−3.61; −0.217], p = 0.027). When further adjusting for comorbidities, the association was no longer present. The full association analysis is presented in Supplemental Table 2. Overall, all associations found were of moderate nature.
Interaction of MPV, arterial stiffness and MPV-related SNPs to survival
During a follow-up period until September 2016 with a median follow-up time of 6.8 [5.5–8.2] years, a total of 580 deaths were registered in the total sample. When stratifying individuals for the sex-specific medians of both MPV and AIx, the best survival was present for the combination of both MPV and AIx below the respective medians (Fig. 3). Cox regression analysis modeling for death including MPV, the interaction term MPV*AIx, and with adjustment for age and sex, heart rate and height, confirmed the association for both MPV and AIx with increased total mortality (Supplemental Table 3). All identified MPV-related SNPs that were related with AIx were tested for an association with total mortality (Table 4). The results of an age and sex adjusted cox-regression model revealed one genetic variant close to ring finger protein 145 gene (RNF145, rs10076782) significantly associated with increased mortality (hazard ratio, 2.02; 95% CI, 1.35 to 3.02; p = 0.00061).
Discussion
To our knowledge, this is a largest population-based study investigating the association between MPV, a potential marker of platelet activity and arterial stiffness. With this study, in a multivariable analysis even after adjustment, we found that there is a positive, strong association between MPV and vascular stiffness in both men and women. An association analysis identified one genetic variant close to ring finger protein 145 gene (RNF145, rs10076782) linked with increased mortality.
MPV and vascular stiffness, biomarker for cardiovascular outcomes
MPV has been previously examined as an index of platelet function and associated to other conventional markers of platelet activation22,23,24. It is known that larger platelets contain more granules and are in general enzymatically and metabolically more active compared to smaller ones exhibiting as a consequence more pro-thrombotic characteristics. Larger platelets secrete more serotonin and beta thromboglobulin and produce more thromboxane A2 than smaller platelets, leading to more vascular complications25.
Vascular stiffness as assessed by the determination of the augmentation index is well validated in large population studies to be a strong predictor of adverse cardiovascular outcomes26,27, and has been incorporated into the current guideline for the management of arterial hypertension as a searching tool for subclinical organ damage28. Since, MPV has been also shown to be associated with increased risk of arterial and venous thrombosis and recent studies indicate that MPV is significantly determined by traditional CVRFs and a higher MPV goes along with a higher risk of death17, it is tempting to conclude that increased MPV may adversely affect vascular function (stiffness) mainly because of its functional “side effects”.
Indeed, the main finding of the present analysis is a positive and strong association between MPV and arterial stiffness, implicating a close link between higher MPV, as potential marker of platelet activation, and increased stiffness of the arterial vessel wall. Differently, platelet count was not associated to arterial stiffness, as previously reported29. With the present study we could also establish that both, a higher MPV as well as increased vascular arterial stiffness are associated with worse survival. Higher MPV and increased arterial stiffness were present in individuals with present CVRFs, particularly diabetes, hypertension and smoking. The finding did not differ between males and females, suggesting that higher MPV is also associated with increased arterial stiffness in females. This may be in contradiction with our previous work on sex specific determinants for MPV in males and females17, where we observed an association of MPV with CVRF in males, but not in females, where MPV was under strong influence of the female hormonal status. However, our previous study addressed the issue of MPV determinants in a wide age range including menstruating, premenopausal and postmenopausal women. Taking the increased incidence of cardiovascular events in females in the postmenopausal period into consideration, MPV might be determined by CVRFs in this group only, which could partly explain the positive association with arterial stiffness found in this study. Indeed, in menopausal women without hormone replacement therapy, diabetes, obesity and smoking were positively associated with MPV (see Supplemental Table 5).
The second important issue concerns the association of MPV-related genetic variants with arterial stiffness. Whereas the majority of studies focused on the association between arterial stiffness and SNPs related with the renin-angiotensin-aldosterone system30, the present studies investigated the association of SNPs related to MPV variability, as already described by GWAS and other works in the literature, with arterial stiffness. Four SNPs in males and one in females were related with AIx in a non-adjusted analysis. After adjustment for age, sex, CVRFs, comorbidities and medication in a multivariable logistic regression model, all observed associations were lost. This fact supports a potential causal relationship between MPV, arterial stiffness and the development of CVD, which needs to be further explored. Further analysis of these common genetic variants and total mortality revealed a strong relation of rs10076782 with increased mortality. Located on chromosome 5, rs10076782 is a genetic variant close to Ring Finger Protein 145 gene (RNF145), which is involved in megakaryopoiesis and platelet formation20. The precise link to arterial stiffness, however, remains still unclear.
Several limitations of this study need to be acknowledged. We did not apply correction for multiple testing for investigating the association of MPV-related genetic variants with arterial stiffness. Although this study was of explorative nature, it was, however, based on the hypothesis of a biological link between platelet activation and arterial stiffness. The hypothetical concept was confirmed by large multivariable models demonstrating a measurable clinical link in humans.
Life style changes in particular exercise and pharmacological interventions are two potential strategies suggested for decreasing arterial stiffness31. The association between MPV, as a marker of platelet reactivity, and arterial stiffness suggests a potential for antiplatelet agents in modulating arterial stiffness. Indeed, a recent study demonstrated that increased platelet reactivity even under clopidogrel medication is associated with impaired arterial stiffness in patients after percutaneous coronary intervention32. Furthermore, increased platelet reactivity was associated with adverse outcome in these patients. Therefore, platelets indeed represent an important player in the pathophysiology of increased arterial stiffness and therefore could act as potential therapeutic target.
In conclusion, with the present study we can demonstrate that MPV is closely and strongly associated with increased arterial stiffness in both males and females in the GHS population and we demonstrate for the first time potential joint genetic variants associated with both traits. Future investigations are necessary to fully understand the molecular processes how activated platelets interact with the vessel wall to cause increased vascular stiffness and what antiplatelet measures are necessary to improve arterial compliance.
Additional Information
How to cite this article: Panova-Noeva, M. et al. Mean Platelet Volume and Arterial Stiffness – Clinical Relationship and Common Genetic Variability. Sci. Rep. 7, 40229; doi: 10.1038/srep40229 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Laurent, S. et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37, 1236–1241 (2001).
Fok, H. & Cruickshank, J. K. Future Treatment of Hypertension: Shifting the Focus from Blood Pressure Lowering to Arterial Stiffness Modulation? Current hypertension reports 17, 67, doi: 10.1007/s11906-015-0569-6 (2015).
Jain, S., Khera, R., Corrales-Medina, V. F., Townsend, R. R. & Chirinos, J. A. “Inflammation and arterial stiffness in humans”. Atherosclerosis 237, 381–390, doi: 10.1016/j.atherosclerosis.2014.09.011 (2014).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874, doi: 10.1038/nature01323 (2002).
Ross, R. Atherosclerosis–an inflammatory disease. The New England journal of medicine 340, 115–126, doi: 10.1056/NEJM199901143400207 (1999).
Morrell, C. N., Aggrey, A. A., Chapman, L. M. & Modjeski, K. L. Emerging roles for platelets as immune and inflammatory cells. Blood 123, 2759–2767, doi: 10.1182/blood-2013-11-462432 (2014).
Karshovska, E. et al. Hyperreactivity of junctional adhesion molecule A-deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circulation research 116, 587–599, doi: 10.1161/CIRCRESAHA.116.304035 (2015).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nature medicine 9, 61–67, doi: 10.1038/nm810 (2003).
Lievens, D. et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 116, 4317–4327, doi: 10.1182/blood-2010-01-261206 (2010).
Nording, H. M., Seizer, P. & Langer, H. F. Platelets in inflammation and atherogenesis. Frontiers in immunology 6, 98, doi: 10.3389/fimmu.2015.00098 (2015).
Wild, P. S. et al. [The Gutenberg Health Study]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 55, 824–829, doi: 10.1007/s00103-012-1502-7 (2012).
Cereda, C. W. et al. Endothelial dysfunction and arterial stiffness in ischemic stroke: the role of sleep-disordered breathing. Stroke; a journal of cerebral circulation 44, 1175–1178, doi: 10.1161/STROKEAHA.111.000112 (2013).
Rubinshtein, R. et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal 31, 1142–1148, doi: 10.1093/eurheartj/ehq010 (2010).
Schnabel, R. B. et al. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circulation. Cardiovascular imaging 4, 371–380, doi: 10.1161/CIRCIMAGING.110.961557 (2011).
Hamburg, N. M. et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 117, 2467–2474, doi: 10.1161/CIRCULATIONAHA.107.748574 (2008).
Patvardhan, E. et al. Augmentation index derived from peripheral arterial tonometry correlates with cardiovascular risk factors. Cardiology research and practice 2011, 253758, doi: 10.4061/2011/253758 (2011).
Panova-Noeva, M. et al. Sex-specific differences in genetic and nongenetic determinants of mean platelet volume: results from the Gutenberg Health Study. Blood 127, 251–259, doi: 10.1182/blood-2015-07-660308 (2016).
Shameer, K. et al. A genome- and phenome-wide association study to identify genetic variants influencing platelet count and volume and their pleiotropic effects. Human genetics 133, 95–109, doi: 10.1007/s00439-013-1355-7 (2014).
Soranzo, N. et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nature genetics 41, 1182–1190, doi: 10.1038/ng.467 (2009).
Gieger, C. et al. New gene functions in megakaryopoiesis and platelet formation. Nature 480, 201–208, doi: 10.1038/nature10659 (2011).
Eicher, J. D. et al. Platelet-Related Variants Identified by Exomechip Meta-analysis in 157,293 Individuals. American journal of human genetics 99, 40–55, doi: 10.1016/j.ajhg.2016.05.005 (2016).
Nadar, S. K., Lip, G. Y. & Blann, A. D. Platelet morphology, soluble P selectin and platelet P-selectin in acute ischaemic stroke. The West Birmingham Stroke Project. Thrombosis and haemostasis 92, 1342–1348, doi: 10.1160/TH04-07-0433 (2004).
Mathur, A., Robinson, M. S., Cotton, J., Martin, J. F. & Erusalimsky, J. D. Platelet reactivity in acute coronary syndromes: evidence for differences in platelet behaviour between unstable angina and myocardial infarction. Thrombosis and haemostasis 85, 989–994 (2001).
Mangalpally, K. K. et al. Platelet activation patterns in platelet size sub-populations: differential responses to aspirin in vitro . Journal of thrombosis and thrombolysis 30, 251–262, doi: 10.1007/s11239-010-0489-x (2010).
Bath, P. M. & Butterworth, R. J. Platelet size: measurement, physiology and vascular disease. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis 7, 157–161 (1996).
Townsend, R. R. et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 66, 698–722, doi: 10.1161/HYP.0000000000000033 (2015).
Laurent, S. et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European heart journal 27, 2588–2605, doi: 10.1093/eurheartj/ehl254 (2006).
Mancia, G. et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European heart journal 34, 2159–2219, doi: 10.1093/eurheartj/eht151 (2013).
Wang, R. T., Li, Y., Zhu, X. Y. & Zhang, Y. N. Increased mean platelet volume is associated with arterial stiffness. Platelets 22, 447–451, doi: 10.3109/09537104.2011.565431 (2011).
Lacolley, P., Challande, P., Osborne-Pellegrin, M. & Regnault, V. Genetics and pathophysiology of arterial stiffness. Cardiovascular research 81, 637–648, doi: 10.1093/cvr/cvn353 (2009).
Wu, C. F. et al. Therapeutic modification of arterial stiffness: An update and comprehensive review. World journal of cardiology 7, 742–753, doi: 10.4330/wjc.v7.i11.742 (2015).
Siasos, G. et al. High platelet reactivity is associated with vascular function in patients after percutaneous coronary intervention receiving clopidogrel. International journal of cardiology 177, 192–196, doi: 10.1016/j.ijcard.2014.09.030 (2014).
Acknowledgements
The Gutenberg Health Study is funded through the government of Rhineland-Palatinate („Stiftung Rheinland-Pfalz für Innovation“, contract AZ 961–386261/733), the research programs “Wissen schafft Zukunft” and “Center for Translational Vascular Biology (CTVB)” of the Johannes Gutenberg-University of Mainz, and its contract with Boehringer Ingelheim and PHILIPS Medical Systems, including unrestricted grants for the Gutenberg Health Study. PSW, SB, TZ and TM are PIs of the German Center for Cardiovascular Research (DZHK). HtC is Fellow of the Gutenberg Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript in its current form. M.P.-N. designed and performed research, analyzed and interpreted data, and wrote the paper; N.A., I.H., J.H.P. contributed to discussion of results and to critical review; A.S. performed the statistical analysis; H.M.S., T.M., K.J.L. and H.t.C. contributed to writing the paper; H.B., N.P., M.B., S.B., T.Z. and J.L. performed research; and P.S.W. designed and performed research, interpreted data and contributed to writing the paper. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Panova-Noeva, M., Arnold, N., Hermanns, M. et al. Mean Platelet Volume and Arterial Stiffness – Clinical Relationship and Common Genetic Variability. Sci Rep 7, 40229 (2017). https://doi.org/10.1038/srep40229
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40229
This article is cited by
-
Mean platelet volume (MPV) as new marker of diabetic macrovascular complications in patients with different glucose homeostasis
Cardiovascular Diabetology (2024)
-
Chronic cigarette smoking is associated with increased arterial stiffness in men and women: evidence from a large population-based cohort
Clinical Research in Cardiology (2023)
-
Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study
Scientific Reports (2021)
-
Mean platelet volume levels in children with sleep-disordered breathing: a meta-analysis
BMC Pediatrics (2020)
-
Platelet counts are associated with arterial stiffness in Chinese Han population: a longitudinal study
BMC Cardiovascular Disorders (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.