Abstract
The double-stranded RNA-binding protein DRB4 of Arabidopsis was shown previously to contribute to the DICER-LIKE 4 (DCL4)-mediated biogenesis of viral small interfering RNAs (vsiRNAs) of 21 nucleotides (nt) in size. However, it is unclear whether all 21-nt vsiRNAs are dependent on this DRB4-DCL4 partnership. To resolve this question, we generated dcl2drb4 and dcl4drb4 double knockout mutants, and subjected them to infections with CPB-CC-PDS, a turnip crinkle virus mutant capable of inducing silencing of the PHYTOENE DESATURASE gene. The dcl2drb4 double knockouts caused a far smaller loss of antiviral silencing than dcl2dcl4. In addition, although both drb4 and dcl4 single mutants permitted a consistent (but small) increase in viral RNA levels, the drb4 mutant correlated with a less pronounced reduction of 21-nt vsiRNAs. Therefore, a substantial subset of DCL4 antiviral activity is DRB4-independent, and may involve other DRB proteins that compensate for loss of DRB4.
Similar content being viewed by others
Introduction
Antiviral RNA silencing in plants enlists DICER-LIKE 2 and 4 (DCL2 and 4), and DCL3 to a lesser extent, to process double-stranded RNA (dsRNA) of virus origin into viral small interfering RNAs (vsiRNAs) of 21–24 nucleotides (nt) in size1,2,3. It was further established that DCL-mediated processing of dsRNA requires the participation of members of the dsRNA-binding protein (DRB) family4,5. For example, processing of endogenous precursor of microRNAs (pre-miRNAs) by DCL1 requires HYL1 (DRB1), whereas processing of the trans-acting siRNA (tasiRNA) precursors by DCL4 requires DRB46,7. More recently it was reported that processing of geminiviral dsRNA by DCL3 requires DRB38. However, the DCL-DRB relationship may not be as specific as initially thought4, as DCL4 processing of tasiRNA precursors other than the tasiRNA3 precursor appears to be less dependent on DRB47. This suggests that either DCL4 could process certain type of dsRNA independent of a DRB, or it also collaborates with other DRBs.
Among the five DRBs encoded by the model plant Arabidopsis, the role of HYL1/DRB1 in DCL1-mediated miRNA biogenesis is best understood6,9,10. On the other hand, DRB4 and DRB3 are the only DRBs found to participate in antiviral silencing2,5,8,11,12. In addition to participating in the biogenesis of vsiRNAs directly, DRB4 has also been shown to play a role in perturbing viral RNA translation13. However, it is important to note that disruption of DRB4 did not lead to a complete loss of 21-nt vsiRNAs2. This suggests that, similar to certain endogenous tasiRNA precursors, some viral dsRNA might also bypass the need for DRB4.
In this report, we attempted to clarify what extent the antiviral function of DCL4 depends on DRB4. To this end, we generated Arabidopsis dcl2drb4 and dcl4drb4 double knockout mutants, and subjected them, along with drb4, dcl2, dcl4 single mutants, and dcl2dcl4 double mutants, to infections with CPB-CC-PDS, a turnip crinkle virus mutant capable of inducing silencing of the PHYTOENE DESATURASE gene. Our results showed that the dcl2drb4 double knockouts caused a far smaller loss of antiviral silencing than dcl2dcl4. In addition, although both drb4 and dcl4 single mutants permitted a consistent (but small) increase in viral RNA levels, the drb4 mutant correlated with a less pronounced reduction of 21-nt vsiRNAs. Therefore, a substantial subset of DCL4 antiviral activity is DRB4-independent, and may involve other DRB proteins that compensate for loss of DRB4.
Results and Discussion
We and others have previously determined that the dcl2dcl4 double knockout mutant of Arabidopsis was nearly completely defective at antiviral silencing against RNA viruses2,14,15. Therefore, if DCL4 requires DRB4 for the entirety of its antiviral function, then the dcl2drb4 and dcl2dcl4 mutants would be predicted to respond to virus attacks in a similar manner. To test this prediction, we generated dcl2drb4, as well as dcl4drb4 double knockouts by crossing dcl2-1 and dcl4-2 with drb4-1. Interestingly, both dcl2drb4 and dcl4drb4 mutants morphologically resembled drb4 single mutant, without the size reduction observed by Nakazawa and colleagues16. Since we also did not observe the dramatic anthocyanin over-accumulation in dcl4 or drb4 single mutants reported by these authors16, we reason that the smaller size of their dcl4drb4 plants might be caused by the specific growth conditions they used. The resulting homozygous double mutants, along with drb4, dcl2, dcl4 single mutants, and dcl2dcl4 double mutants, were subjected to infections with CPB-CC-PDS which is a turnip crinkle virus (TCV) mutant encoding an attenuated suppressor of RNA silencing contains an insertion of 90 nucleotides (nt), derived from the PHYTOENE DESATURASE (PDS) gene of Arabidopsis. The PDS insert induces modest PDS silencing in CPB-CC-PDS-infected plants, providing a visual indicator for the silencing-inducing capability of this viral mutant16. Wild type TCV was not used because its strong silencing suppressor obscures the functional manifestation of plant mutants2,14.
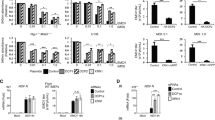
The CPB-CC-PDS-infected plants were reared in growth chambers with a constant temperature of 18 °C, and 14 hour daylight. As shown in Fig. 1A, plants photographed at 25 days post inoculation (dpi) showed varying degrees of PDS silencing along the secondary veins depending on the mutant background. It is worth noting that the extent of PDS silencing in drb4 plants was almost indistinguishable from wildtype Col-0 plants (Fig. 1A, compare panels 2 and 3), despite a small but consistent increase in viral RNA levels in both inoculated and systemic leaves (IL and SL; Fig. 1B, compare lanes 5, 6 with 3, 4). In contrast, dcl2 mutant plants showed substantially more intense PDS silencing than both drb4 and Col-0 (Fig. 1A, compare panels 2, 3, and 4), despite a much smaller increase in viral RNA levels (Fig. 1B, IL and SL, compare lanes 7, 8 with 5, 6). Therefore, unlike in dcl2, increased viral RNA accumulation in drb4 did not result in enhanced target silencing. This suggests that in addition to contributing to DCL4-mediated dsRNA processing, DRB4 might also be needed downstream for vsiRNA-guided slicing of complementary mRNAs.
Loss of antiviral silencing activity in dcl2drb4 double knockouts is substantially less debilitating than in dcl2dcl4 mutant.
(A) PDS silencing induced by CPB-CC-PDS infection of various Arabidopsis mutant plants. Images were recorded at 25 dpi. (B) Northern blot hybridization of the total RNAs extracted from the plants shown in A. Each RNA sample was extracted from six inoculated or systemic leaves (IL or SL) pooled from six plants. To further even out variabilities, two different RNA samples were analyzed per treatment. The probe was a mix of five 32P-labelled oligos complementary to TCV genomic RNA (gRNA). EB: ethidium bromide-stained Northern gel. sgRNA: subgenomic RNA. (C). Northern blot hybridization of vsiRNAs. See Qu et al.2 for a detailed procedure.
We also examined the vsiRNA profiles in these plants. In agreement with our previous studies15, the CPB-CC-PDS-infected Col-0 plants accumulated predominantly DCL4-dependent 21-nt siRNAs accompanied by a barely detectable 22-nt band (Fig. 1C, lane 2). The 20-nt band near the bottom of lanes 2, 4, and 5 likely have derived from 21-nt vsiRNAs, as it was absent in dcl4 and dcl4drb4 plants (lane 6 and 7). In drb4, both the 20-nt and 21-nt vsiRNA bands were weakened, accompanied by a visible enhancement of the 22-nt band. This suggests that DRB4 does act similarly as DCL4 in that its disruption led to reduction of 21-nt vsiRNAs and increase of 22-nt vsiRNAs. However, in drb4 plants the reduction of 21-nt vsiRNAs and its 20-nt derivative was much less dramatic than in dcl4 plants, and the corresponding increase of 22-nt vsiRNAs was also much less pronounced. Therefore, DRB4 is needed for the biogenesis of some, but not all, vsiRNAs produced by DCL4. Conversely, DCL4 is capable of processing certain types of viral dsRNAs into 21-nt vsiRNAs independently of DRB4.
Further strong support for the existence of a DRB4-independent subset of DCL4 activity came from examining dcl2drb4 mutant plants. As shown in Fig. 1A, panel 5, the CPB-CC-PDS-infected dcl2drb4 showed a PDS silencing pattern that is slightly more intense than dcl2 single mutant, as evidenced by slightly wider and more intense bleaching of secondary veins. More tellingly, the viral RNA levels in these plants were also only slightly increased compared with drb4 or dcl2 single mutants (Fig. 1B, IL and SL, lanes 9 and 10). All these findings are in stark contrast with the dcl2dcl4 mutants, where high levels of CPB-CC-PDS RNA accumulation caused much more severe viral symptoms that eventually led to the death of many plants, but with no PDS silencing15 (also see Fig. 1A, panel 8, and Fig. 1B, lanes 15 and 16). Therefore, DCL4, or at least a substantial fraction of it, was still functional in the dcl2drb4 mutant, and acted potently to destruct viral RNA. Indeed, there was actually a modest increase of 21-nt vsiRNA levels in the dcl2drb4 mutant than in dcl2 single mutant (Fig. 1C, lane 4 and 5), probably reflecting the small increase in the level of viral RNA that could serve as templates for DCL4.
Finally, consistent with a potential role of DRB4 in a downstream step13, PDS silencing was actually less intense in dcl4drb4 double mutant than in dcl4 single mutant (Fig. 1A, panels 6 and 7), despite similar levels of viral RNA in the two kinds of plants (Fig. 1B, lanes 11–14), as well as similar patterns of vsiRNAs (Fig. 1C, lanes 6 and 7). Together our results confirmed a critical role of DRB4 in DCL4-mediated 21-nt vsiRNA biogenesis and antiviral defense, as evidenced by a modest increase in viral RNA levels, and a detectable decline of 21- and 22-nt vsiRNA ratio in virus-infected drb4 plants. These changes are similar to, but less penetrating than, in dcl4 plants, suggesting that DRB4 and DCL4 participate in the same vsiRNA-producing pathway. Surprisingly however, DCL4 could accomplish most of its antiviral function in the absence of DRB4, as knocking out DRB4 in dcl2 background (as in dcl2drb4) had a far smaller effect than knocking out DCL4 itself (as in dcl2dcl4) on viral RNA accumulation, target silencing, and disease symptoms. How do we explain these findings? We reason that while DRB4 clearly has a unique role in the biogenesis of a subset of 21-nt vsiRNAs, the function of these vsiRNAs could be readily be compensated by other 21-nt vsiRNAs produced without the involvement of DRB4. Although it is possible that DCL4 could process certain types of viral dsRNAs without any DRBs, it appears to be more plausible that one or more other members of the five-member DRB family could substitute for the loss of DRB4 and assist DCL4 in 21-nt vsiRNA biogenesis. Our next goal will be identify these additional DRBs that contribute to antiviral silencing against RNA viruses.
Materials and Methods
Construct
CPB-CC-PDS, has been described in a previous study15. Briefly, it differs from wildtype TCV at two positions: (i) it contains an arginine (R) to threonine (T) mutation at position no. 130 of TCV capsid protein (CP) that substantially compromised the ability of TCV CP to suppress RNA silencing14; (ii) it additionally contains an insertion of 90 nucleotides (nt), derived from the PHYTOENE DESATURASE (PDS) gene of Arabidopsis. The PDS insert, located immediately downstream of the CP coding region, induces modest PDS silencing in CPB-CC-PDS-infected plants, providing a visual indicator for the silencing-inducing capability of this viral mutant15.
Plant materials
The sources of Col-0, dcl, drb4 mutant plants have been described previously2. dcl2drb4, as well as dcl4drb4 double knockouts were generated by crossing dcl2-1 and dcl4-2 with drb4-1 respectively. Uninfected Arabidopsis plants were reared in a growth room set at 20 °C, with 14 h of daylight. After inoculation with in vitro transcripts of TCV derivative- CPB-CC-PDS, the CPB-CC-PDS-infected plants were moved into Adaptis A1000 growth chambers (Conviron, Winnipeg, Manitoba,Canada),with temperature set at 18 °C. Inoculated leaves (IL) samples were collected at 5 days post inoculation(dpi) while upper young leaves (UL) were collected at 10 dpi for analysis. Leaves from six different plants, one leaf per plant, were pooled before RNA or protein extractions to minimize the sampling errors.
RNA blot analysis
Total RNAs were extracted from infected plants and subjected to RNA blot analysis to detect TCV viral RNAs, or vsRNAs using protocols outlined in previous reports2. 0.8 μg of Arabidopsis RNA were used to detect viral RNA, while for vsRNAs detection 10 μg of Arabidopsis RNA were used. The probes were a mixture of five different oligonucleotides end labeled with 32P.
Additional Information
How to cite this article: Zhang, X. et al. Incomplete DRB4-dependence of the DCL4-mediated antiviral defense. Sci. Rep. 6, 39244; doi: 10.1038/srep39244 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ding, S. & Lu, R. Virus-derived siRNAs and piRNAs in immunity and pathogenesis. Curr. Opin. Virol. 1, 533–544 (2011).
Qu, F., Ye, X. & Morris, T. J. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc. Natl. Acad. Sci. USA 105, 14732–14737 (2008).
Wang, M., Weiberg, A. & Jin, H. Pathogen small RNAs: a new class of effectors for pathogen attacks. Mol. Plant Pathol. 16, 219–223 (2015).
Hiraguri, A. et al. Specific interactions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol. Biol. 57, 173–188 (2005).
Curtin, S. J. et al. The roles of plant dsRNA-binding proteins in RNAi-like pathways. FEBS Lett. 582, 2753–2760 (2008).
Han, M. H., Goud, S., Song, L. & Federoff, N. The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc. Natl. Acad. Sci. USA 101, 1093–1098 (2004).
Adenot, X. et al. DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr. Biol. 16, 927–932 (2006).
Raja, P., Jackel, J. N., Li, S., Heard, I. M. & Bisaro D. M. Arabidopsis double-stranded RNA binding protein DRB3 participates in methylation-mediated defense against geminiviruses. J Virol. 88, 611–2622 (2014).
Vasquez, F., Gasciolli, V., Crete, P. & Vaucheret, H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 14, 348–351 (2004).
Kurihara, Y., Takashi, Y. & Watanabe, Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA. 12, 206–12 (2006).
Haas, G. et al. Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J. 27, 2102–2112 (2008).
Fukudome, A. et al. Specific requirement of DRB4, a dsRNA-binding protein, for the in vitro dsRNA-cleaving activity of Arabidopsis Dicer-like 4. RNA 17, 750–760 (2011).
Jakubiec, A., Yang, S. W. & Chua, N. H. Arabidopsis DRB4 protein in antiviral defense against Turnip yellow mosaic virus infection. Plant J. 69, 14–25 (2012).
Cao, M. et al. The Capsid protein of Turnip crinkle virus overcomes two separate defense barriers to facilitate systemic movement of the virus in Arabidopsis. J. Virol. 84, 7793–7802 (2010).
Zhang, X., Zhang, X., Singh, J., Li, D. & Qu, F. Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires DCL2, AGO2, and HEN1. J Virol. 86, 6847–6854 (2012).
Nakazawa, Y., Hiraguri, A., Moriyama, H. & Fukuhara, T. The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Mol. Biol. 63, 777–785 (2007).
Acknowledgements
We thank members of Qu lab for stimulating discussions, and Drs. Peg Redinbaugh and Lucy Stewart for generous equipment sharing. This study was supported by National Natural Science Foundation of China (31570145, 31201264), a seed grant from Ohio Agricultural Research and Development Center. X.C. Zhang was supported in part by a scholarship from China Scholarship Council (201303260010).
Author information
Authors and Affiliations
Contributions
X.-F.Z., X.Z. and K.W. performed the experiments. Z.L. D.L and F.Q. conceived the study. X.Z. and F.Q. interpreted the data and wrote the manuscript. All authors reviewed and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, X., Zhang, X., Wu, K. et al. Incomplete DRB4-dependence of the DCL4-mediated antiviral defense. Sci Rep 6, 39244 (2016). https://doi.org/10.1038/srep39244
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39244
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.