Abstract
Understanding of T helper 17 lineage (TH17) polarization has been significantly promoted by cell culture experiments that reduce the complexity of the in vivo environment. We here investigated TH17 amplification by coating of cytokine preparations. Cytokine preparations coated to the surface compared to the same amount given in solution significantly enhanced TH17 polarization assessed by flow cytometry and interleukin (IL)-17A, IL-17F and RORγt mRNA expression. T cell proliferation and TH1 polarization were similarly enhanced while TREG polarization was impeded. TH17 amplification was replicated by coating the plate with low amounts of FCS or albumin as used as carrier protein for cytokines (0.5 μl 0.1%). It was unaltered by filtration, protein digestion and arylhydrocarbon receptor blockade, not replicated by LPS and independent of integrin stimulation. TH17 amplification required anti-CD3 stimulation and was T cell intrinsic. Supernatants of CD4+ cells polarized on coated cytokine preparations with carrier albumin conferred amplification to fresh splenocytes. Coating markedly elevated CD4+ IL-22 mRNA expression and IL-22 blockade significantly reduced TH17 amplification. Our data show TH17 amplification by coated albumin in the low amounts present in recombinant cytokine preparations. This unexpected adjuvant like effect underscores the need for controls also for temporal and spatial factors in cell culture.
Similar content being viewed by others
Introduction
In vitro culture is a standard method to investigate mechanisms of T helper cell polarization and efficacy of therapeutic interventions targeting T helper cell subsets1,2,3. T cells are activated by stimulation through T cell receptor (TCR) interactions with cognate major histocompatibility complex molecules and co-stimulation via CD284.
Polarization to specific T helper lineages requires cytokines in addition to T cell receptor stimulation. Transforming growth factor beta (TGFβ), interleukin (IL)-6 and IL-23 promote murine T helper 17 cell lineage (TH17) polarization5,6,7,8. STAT3 and RORγt transcription factors promote TH17 signature cytokine IL-17A and IL-17F gene expression9. STAT3 can be activated by IL-21 and IL-22, a member of the IL-10 family. Both IL-21 and IL-22 are expressed in TH17 cells under specific conditions10,11,12,13,14,15.
In addition to cytokines, a number of other agents modulate TH17 polarization1,2,3. For example, low molecular weight ligands to the aryl hydrocarbon receptor (AhR) are found in high concentrations in Iscove’s modified Dulbecco’s (IMDM) medium and are therefore common cell culture ingredients16,17. AhR activation induces a marked increase in TH17 cell proportion and cytokine production18,19,20. Lipopolysaccharide (LPS), a component of gram-negative bacteria, is a common contaminant of recombinant protein preparations21. Its role in TH17 cell polarization is controversial. While high concentrations increased TH17 polarization in vitro22 and in vivo23, a lower concentration was without effect24. Enhancement of T cell proliferation is the intended effect of vaccine adjuvants in vivo, some of which directly stimulate T cells25. Beyond enhancing antigen specific response, some adjuvants favor distinct TH lineages, for example, alum induces an innate response that promotes TH2 polarization26,27. Regarding TH17 polarization28, complete Freund’s adjuvant (CFA), a water-in-oil emulsion with heat-killed mycobacteria induces IL-17 secreting cells in vivo. The in vitro effect has not been reported.
Interaction with the vascular wall and other surfaces, for example via integrins and activation of the cytoskeleton modifies T cell response29. Beyond this, integrins can promote TH17 differentiation by binding an RGD peptide sequence in TGFβ30,31. Fractalkine, the unique ligand of CX3CR1, is a stalked cytokine that exists in soluble and surface bound form in vivo and modulates immune cell migration and function32. Fractalkine effects in vitro have largely been studied using surface-bound recombinant cytokine33,34,35. Fractalkine receptor CX3CR1 is expressed on T cells36 including TH1 cells37,38. We recently demonstrated its expression on both TH17 and TREG cells and induction by TGFβ during lymphocyte culture39. This led us to investigate the effect of coated and soluble recombinant fractalkine in TH17 cell polarization. Performing controls with specific receptor deficient cells revealed a receptor-unspecific TH17 amplification loop by diverse coated versus soluble recombinant cytokine preparations.
To define appropriate controls for further TH17 polarization experiments, where specific gene deficient controls might not be available, we here explored the underlying mechanism.
Results
Amplification of TH17 polarization by a coated fractalkine preparation is receptor independent
Given the effect of fractalkine receptor CX3CR1 on T cell polarization demonstrated by others37 and impeded TH17 polarization in specific gene deficient cells found by us39, we investigated the effect of recombinant fractalkine on TH17 polarization in vitro. Coated recombinant fractalkine preparation from one, but not another vendor markedly increased TH17 polarization (Fig. 1a–c). However, this effect was also observed in CX3CR1−/− cells included as a specificity control, suggesting an unspecific effect of coated substance. Similarly, a coated, but not soluble IL-17A preparation markedly enhanced TH17 polarization of both wildtype and IL-17 receptor A deficient (Il17ra−/−) splenocytes (Suppl. Fig. 1A). Again, this effect appeared only for one of the tested preparations.
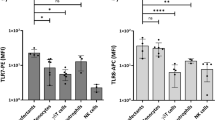
Effect of coated versus soluble fractalkine preparations on TH17 cell polarization in CX3CR1−/− cells.
(a–c) Wildtype and fractalkine receptor deficient (CX3CR1−/−) cells were subjected to TH17 polarization with IL-6, TGFβ and IL-23 in the absence and presence of coated (“coat”) and soluble (“sol”) recombinant fractalkine at 20 and 100 nM final concentrations. The proportion of TH17 cells was assessed by intracellular IL-17A staining after re-stimulation on day 4 (a, examples, b statistical analysis of fractalkine from Peprotech, and c, from R&D Systems, n = 4–8 from 2–4 indep. exp.).
This marked receptor-independent effect led us to further investigate its mechanism in order to avoid unspecific findings.
Cytokine coating to the cell culture surface amplifies TH17 and TH1, but not TREG polarization
We investigated the effect of coating of cytokine preparations used for polarization of TH1, TH17 and TREG cells in parallel (see methods and Suppl. Fig. 6 for detailed protocols). Pre-coating the plate with TH17 polarizing cytokines IL-6, IL-23 and TGFβ amplified TH17 polarization compared to the same amount given to the cell culture medium (Fig. 2a). A similar effect was observed for TH1 polarization (Fig. 2b). In contrast, TREG polarizing agents in coated form decreased the proportion of TREG cells (Fig. 2c). In parallel, coated but not soluble IL-17A inhibited both wildtype and Il17ra−/− TREG polarization (Suppl. Fig. 1B). T cell proliferation assessed by CFSE dilution was increased by coating in all tested conditions (Fig. 2d–f). There was no effect of coating on the proportion of live T cells among all events recorded after restimulation in either TH17 or TREG conditions, while a minor decrease in the TH1 condition was noted (Suppl. Fig. 2A–C). However, the proportion of live cells among all T cells was not affected for either lineage (Suppl. Fig. 2D,E). TH17 polarization induced a significant increase in CD44 and loss of CD62l, this was however not significantly altered by coating (Suppl. Fig. 3).
Recombinant cytokine preparations coated to the cell culture plate increase T cell proliferation and amplify TH17 and TH1, but not TREG polarization.
For T helper cell polarization in total splenocytes on anti-CD3 and anti-CD28 antibodies, cytokines were either added to the cell culture medium in soluble form (“sol”) at standard or elevated concentration or coated to the cell culture vessel beforehand (“coat”) (see methods and Suppl. Fig. 6 for details). (a–c) T helper cell polarization was assessed by intracellular staining for IL-17A (TH17, A, n = 14, 7 exp.), IFN-γ (TH1, B, n = 6, 3 exp.) after restimulation on day 4 of polarization with IL-6 (50 ng/ml), TGFβ (1 ng/ml), IL-23 (20 ng/ml) or 10 ng/ml IL-12, respectively, or 5x these amounts as indicated. TREG polarization was assessed by staining for FoxP3 on day 3 of polarization with TGFβ (10 ng/ml) and IL-2 (10 ng/ml) or 5x these amounts as indicated. (C, n = 10, 5 exp., examples in A-C are 1x soluble and 5x coated experiments). (d–f) Proliferation was assessed by CFSE dilution in TH17 (d), TH1 (e) and TREG (f) polarized cells (n = 6 each, 3 indep. exp.). (g) The effect of coating with individual cytokines was investigated on day 4 of TH17 polarization (n = 4, 2 indep. exp.). (h–j) Expression of IL-17A (h) and IL-17F (i) and the TH17 signature transcription factor RORγt (j) was measured by qPCR on day 4 of polarization (n = 4, 2 indep. exp.).
Amplification of TH17 polarization required all TH17 polarizing cytokines TGFβ, IL-6 and IL-23 (data not shown), but was obtained if any single one of them was coated to the plate while the others were in solution (Fig. 2g). TH17 polarization was further investigated by qPCR. Pre-coated preparations significantly increased IL-17A, IL-17F and RORγt gene expression during TH17 polarization (Fig. 2h–j). Similarly, in CX3CR1−/− cells, coating with a recombinant fractalkine preparation enhanced RORγt in TH17 cells, with much less effect on Tbet and FoxP3 (Suppl. Fig. 4). TH2 marker transcription factor GATA3 and cytokines IL-4 and IL-5 were not significantly affected in the tested lineages (Suppl. Fig. 5).
These data depict an application dependent, non-cytokine-specific amplification of TH17 cells by commercial cytokine preparations.
Recombinant cytokine preparations amplify TH17 polarization in an adjuvant like fashion
Information on cytokine sources and specification is summarized in Suppl. Table 1. We contacted all suppliers for a complete list of ingredients and their concentrations, however, did frequently not obtain complete information. LPS is a known contaminant of recombinant cytokines. A large dose range that included values markedly above the maximal doses claimed for the preparations (below 1 or 0.1EU/μg) was tested in TH17 polarization (Suppl. Figs 6 and 7A). Neither coated nor soluble LPS affected the polarization. Also, integrin stimulation with collagen or RGD peptide had no effect (Suppl. Fig. 7B).
AhR ligands that are abundant in IMDM compared to RPMI favor TH17 polarization16. When the effect of coating was tested in direct comparison of both media, the proportion of TH17 cells was higher in IMDM. However, the effect of coated versus soluble preparations was observed in both (Fig. 3a). This additive effect argues against a direct AhR stimulation by components of the cytokine preparations. More specifically, AhR agonist FICZ in coated form dose dependently increased TH17 polarization, but did not reach the level observed for coated cytokine preparations. Conversely, AhR blocker CH-223191 did not impair TH17 amplification by coated preparations (Fig. 3b).
TH17 amplification is independent of AhR stimulation and also observed after culture on coated CFA.
(a) TH17 polarization with and without cytokine coating in AhR ligand rich (IMDM) versus low (RPMI) cell culture media for 4 days (n = 8 from 4 indep. exp., Bonferroni after ANOVA). (b) TH17 polarization with soluble cytokine in the presence of coated AhR agonist FICZ at the indicated doses and antagonist CH-223191 on coated cytokine preparation (n = 6 from 3 indep. exp., Bonferroni of selected conditions after ANOVA). (c) Cell culture plate coating with TH17 polarizing cytokines was performed with or without trypsin digestion as described in methods. (4 days, n = 4 from 2 indep. exp.). (d–g) CFA (d,e) and Montanide (Mon, f,g) water-in-oil adjuvants were added in solution or coated to the plate during TH17 polarization and the proportion of TH17 cells (d,f) and the mean TCR expression on live T cells (e,g) determined (4 days, n = 6–8, 3–4 indep. exp., Bonferroni after ANOVA). For all TH17 polarizations cytokines were added at standard concentration (50 ng/ml IL-6, 1 ng/ml TGFβ, 20 ng/ml IL-23) to the media of all cells, coating with 5x of these concentrations was as indicated in panels A and B, all cells were restimulated with PMA/ionomycin.
Proteome analysis of an IL-17A preparation that amplified TH17 in coated, but not soluble form, compared to one that did not, showed mostly plasma and keratin components in both and no obvious candidates for TH17 polarization (Suppl. Table 2). Functionally, trypsin digestion of the coated proteins did not abrogate TH17 amplification by coating (Fig. 3c) while it significantly decreased soluble cytokine polarizing function (Suppl. Fig. 7D). These effects are consistent with an adjuvant effect. CFA is one of the strongest known adjuvants with a known TH17 favoring function in vivo40,41,42. In vitro, CFA enhanced TH17 polarization in coated, but not soluble form (Fig. 3d), albeit to a somewhat lesser degree than coated cytokine preparations. In direct comparison, a new generation of water-in-oil emulsion adjuvant, Montanide ISA51VG, was investigated43. Coated Montanide did not significantly enhance TH17 polarization (Fig. 3f). Both adjuvants, however, induced much stronger TCR downregulation in coated than soluble form (Fig. 3e,g), a feature of combined CD3 and T cell receptor stimulation44.
These results indicate an adjuvant-like amplification of TH17 polarization by coated cytokine preparations.
TH17 amplification requires anti-CD3 stimulation and is replicated by coating with albumin
To study the role of T cell receptor stimulation in the TH17 amplification caused by cytokine preparations, we next investigated the protocol of anti-CD3 and anti-CD28 stimulation. As a standard, these antibodies were coated to the culture plate (Suppl. Fig. 6). TH17 amplification depended on anti-CD3 but not anti-CD28 antibody (Fig. 4a). It was preserved with a different ultrapure anti-CD3 preparation (n = 2, data not shown). Giving anti-CD3 antibody and cytokines simultaneously into solution was equivalent to coating both to the plate (Fig. 4b). A rat IgG isotype to the anti-CD3 antibody was used as control. It did not amplify TH17 polarization (Fig. 4c). T cell receptor (TCR) surface expression significantly decreased with cytokine coating and also simultaneous addition of cytokines and anti-CD3 antibody (Fig. 4d). As a standard, we used highly adsorbent tissue culture plasticware and therefore hypothesized that pre-adsorbed anti-CD3 molecule interaction with T cells and cytokine preparations might be limited. Indeed, previous surface blocking with FCS (Fig. 4e) and use of sterile low absorbent polystyrene and polypropylene flow cytometry tubes (data not shown) rendered coated and soluble cytokine preparations equipotent in TH17 amplification. On the other hand, these results might suggest that spatial proximity or even direct interaction of the anti-CD3 antibody with a component of coated FCS was amplifying TH17 differentiation. Albumin is a main part of serum. Indeed, also lower amounts of coated FCS (0.5 μl 10% in PBS) and low amounts of albumin (BSA) significantly amplified TH17 polarization (Fig. 4f). This range of BSA was present as a carrier protein in the recombinant cytokine preparations. While coated BSA in the amount of the cytokine itself did not alter TH17 polarization (Suppl. Fig. 7D), 0.5 μl of 0.1% BSA as used as carrier indeed amplified TH17 polarization in coated but not soluble form (Fig. 4g). TH17 amplification persisted after an additional filtration step (Fig. 4g). Thus, coated albumin completely replicated the receptor independent effects of coated cytokine preparations that initially prompted our study (Fig. 1, Suppl. Fig. 1).
Anti-CD3 antibody in conjunction with FCS or albumin amplifies TH17 polarization.
TH17 polarization with coated versus soluble cytokine preparations in the absence and presence of anti-CD3 and anti-CD28 antibodies (n = 6, 3 exp., Bonferroni after ANOVA). (b) Addition of anti-CD3 and anti-CD28 together with coated and soluble preparations (n = 6, 3 indep. exp., Bonferroni after ANOVA). (c) Addition of IgG isotype (n = 6, 3 indep. exp., Bonferroni after ANOVA). (d) αβTCR expression on the T cell surface after TH17 polarization with addition of anti-CD3 and anti-CD28 antibodies together with cytokines (IL-6, IL-23 and TGFβ) in solution or coated form (n = 6 from 3 indep. exp.). (e) Pre-adsorption of the cell culture plate with 10% FCS (n = 4, 2 indep. exp., Bonferroni after ANOVA). (f) Effect of coating cell culture grade FCS (0.5 μl 10% in PBS) or 0.1% BSA (0.5 μl 0.1%, n = 4, 2 indep. exp). (g) Coating with 0.5 μl and 1.5 μl 0.1% BSA as used as carrier protein with and without an additional filtration step (0.2 μm, n = 8, 4 indep. exp., Bonferroni after ANOVA). For TH17 polarizations, cytokines were added at 1x concentration (50 ng/ml IL-6, 1 ng/ml TGFβ, 20 ng/ml IL-23) to the media of all cells, coating with 5x of these concentrations is indicated in the legends, polarizations were conducted for 4 days and all cells were restimulated with PMA/ionomycin.
These results identify coated albumin as amplifier of TH17 polarization and are consistent with a combined effect of albumin and the anti-CD3 antibody.
TH17 amplification is T cell intrinsic and induces IL-22 production
To investigate whether the observed effect was T cell intrinsic, magnetically enriched CD4+ splenocytes were polarized with coated or soluble cytokines containing carrier albumin. The TH17 amplification conveyed by coating persisted (Fig. 5a), also with a negative selection method from a different manufacturer (data not shown) and also in negatively selected naïve CD4+ T cells (Fig. 5b). To determine whether T cells secreted a soluble factor that amplified their own polarization, we treated splenocytes during TH17 polarization with supernatants from CD4+ and CD4− cells that had been cultured with either soluble or coated cytokines. Supernatants from CD4+ much more than CD4− splenocytes cultured on coated cytokines conferred TH17 amplification (Fig. 5c). These data are consistent with a CD4+ autocrine loop enhancing TH17 polarization.
TH17 amplification by coated cytokine preparations containing albumin carrier is T cell intrinsic and induces IL-22.
(a,b) TH17 polarization of CD4+ (A) and naïve CD4+ T cells (B) on coated and with soluble cytokine preparations (n = 4, 2 indep. exp. each). (c) TH17 polarization of splenocytes cultured with the supernatants of CD4+ enriched or CD4+ depleted splenocytes that had been stimulated with either coated or soluble TH17 cytokines (n = 8, 4 indep. exp., Bonferroni after ANOVA). (d,e) Expression of IL-21 (D) and IL-22 (e) was measured by qPCR on day 3 of culture of CD4+ and CD4− enriched splenocytes without exogenous cytokines (TH0) and with either coated or soluble TH17 polarizing cytokines (n = 6, 3 indep. exp.). (f) Anti-IL-22 antibody or isotype control was applied to TH17 polarization of splenocytes stimulated with supernatants from CD4+ cells grown on coated TH17 polarizing cytokines (n = 4, 2 indep. exp., TH17 polarizations were conducted for 4 days, cytokines were added at 1x concentration (50 ng/ml IL-6, 1 ng/ml TGFβ, 20 ng/ml IL-23) to the media of all cells, coating with 5x of these concentrations is indicated in the legend of panel A–E, cells were restimulated with PMA/ionomycin for flow cytometric analysis).
We next investigated IL-21 and IL-22, cytokines that can be produced by TH17 cells and can both activate STAT3, a TH17 enhancing transcription factor14,15. While IL-21 gene expression was decreased in CD4+ cells on coated cytokine preparations compared to cytokines in solution, IL-22 was significantly upregulated (Fig. 5d,e). To test for a function of secreted IL-22, TH17 polarization was performed in the presence of supernatant of CD4+ cells polarized on coated cytokines as in Fig. 5c, but with blockade of IL-22 or isotype control (Fig. 5f). IL-22 receptor mRNA expression was detectable in splenocytes after culture on coated preparations (0.06 ± 0.00% of HPRT, n = 2, data not shown). Indeed, IL-22 blockade significantly reduced TH17 amplification by supernatants of CD4+ T cells stimulated with coated cytokine preparations.
This indicates that IL-22 contributes to TH17 amplification by coated cytokine preparations containing carrier albumin.
Discussion
Our data show significant amplification of in vitro TH17 polarization if low amounts of albumin or cytokine preparations containing carrier albumin are coated to the plate in conjunction with anti-CD3 antibody. The amplification was mediated in an autocrine fashion and also increased IL-22 production.
TH17 polarization required IL-6, IL-23 and TGFβ cytokines in any of the tested conditions, but was markedly enhanced if these or the carrier protein only were coated to the plate. Enhancement of TH17 polarization required combined action of the anti-CD3 antibody and albumin or a cytokine preparation containing it, possibly, but not necessarily in coated form. In addition, coating induced T cell proliferation and a marked loss of TCR surface expression in all studied conditions. This is consistent with TCR activation44 and TCR-CD3 complex formation that promotes T cell proliferation45.
The vaccine adjuvant CFA in coated form similarly enhanced TH17 polarization, together with downregulation of TCR surface expression. Beyond amplification of the immune response, some vaccine adjuvants favor individual T helper cell lineages25. For CFA, in vivo measurements have demonstrated elevated IL-17A, IL-10 and IL-22 cytokine expression in CD4+ T cells under TH17 polarizing conditions40. Also, an early report before discovery of IL-17A describes a marked increase in myelopoiesis, a hallmark of IL-17A induced G-CSF production, after CFA treatment46. The effect of coated albumin or carrier containing cytokine preparations in amplifying TH17 cells was rather stronger than for CFA in our experiments. Also, the albumin effects were completely resistant to digestion with trypsin, and indeed, understanding of mechanisms of most vaccine adjuvants functions is incomplete at present25.
At the same time of TH17 and TH1 amplification, TREG polarization was impaired by coated cytokine preparations containing albumin carrier. While TH17 and TREG cells share a common requirement of TGFβ for polarization47, a high TGFβ concentration induces the TREG defining transcription factor FoxP3 that represses RORγt47,48. A large number of other factors regulating gene expression including mammalian target of rapamycin (mTOR), hypoxia inducible factor 1 alpha (HIF1α) and retinoic acid receptor alpha (RORα) are also involved in this reciprocal regulation1,2,3. TREG cells limit response to vaccines, for example the effectiveness of BCG vaccination against M. tuberculosis49. Therefore, adjuvants favoring TH1 and TH17 at the cost of TREG cells might be helpful. However, decreasing TREG function has a strong deleterious potential and needs to be tested with great caution50.
IL-22 was increased during TH17 polarization on coated cytokines containing albumin carrier and was at least partly responsible for autocrine amplification, possibly by STAT3 activation14. TGFβ suppresses IL-22 expression51 at the concentration of 1 ng/ml that is commonly used for TH17 polarization and also in our experiments47. This restriction was apparently removed in cells on coated cytokine preparations. Similar mechanisms may contribute to IL-17A and IL-22 co-expression in vivo52,53,54. IL-22 is highly relevant biologically. For example, it promotes wound healing in a large range of conditions13,14,15,55. In other settings, co-expression of IL-22 together with IL-17A promoted airway53 and chronic liver inflammation and fibrosis in hepatitis B virus infected patients and HBV transgenic mice56 and expression of antimicrobial peptides in human keratinocytes54. Our data indicate that direct IL-22 effects also need to be considered when in vitro polarized TH17 cells are adoptively transferred to investigate their roles in disease models in vivo.
In summary, our data show that TH17 polarization is significantly amplified and IL-22 expression increased by a combined action of coated albumin or cytokine preparations containing it as carrier and anti-CD3 antibody that can be inadvertently caused by in vitro culture conditions. This adjuvant-like effect on TH17 amplification adds a new degree of complexity to TH17 polarization in vitro and possibly alters TH17 function in immune disorders in vivo.
Methods
Animals
Wild-type (wt) C57Bl/6, CX3CR1−/− ( = CX3CR1gfp/gfp 57) (Jackson Labs, Bar Harbor, ME) and Il17ra−/− mice52 all on C57Bl/6 background, were genotyped by PCR. Mice were kept in specific-pathogen-free conditions. Harvest of primary murine cells was approved by the Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Lower Saxony, Germany according to the current regulations. All methods were performed according to the relevant guidelines.
Cell culture, stimulation and T cell polarization
T cell culture was performed in 96 well plates (Nunclon Delta Surface, Thermo Fisher Scientific, Waltham, MA, USA), unless sterile polystyrene (Falcon, Thermo Fisher Scientific) or polypropylene (Sarstedt, Nürmbrecht, Germany) tubes were used as indicated. Cultures were in complete IMDM (Gibco, Thermo Fisher Scientific) with 10% fetal calf serum (FCS, PAN Biotech, Aidenbach, Germany) and penicillin (10,000 U/ml)/streptomycin (10,000 μg/ml) (Gibco, Waltham, MA) unless otherwise indicated.
Total and CD4+ enriched or depleted (CD4 L3T4, MicroBeads, Miltenyi, Bergisch Gladbach, Germany or MojoSortTM Mouse CD4 T cell isolation kit, Biolegend, San Diego, CA, USA, each applied according to the manufacturer’s instructions, reaching above 90% purity) mouse splenic lymphocytes were cultured at a concentration of 5 × 106/ml. MojoSortTM Mouse CD4 Naïve T Cell Isolation Kit (Biolegend, San Diego, CA, USA) was used as directed by the manufacturer. Cultures in complete IMDM without exogenous cytokines are depicted as TH0. TH17 polarization was performed with anti-IFN-γ and anti-IL-4 (both 3 μg/ml) and IL-6 (50 ng/ml), TGFβ (1 ng/ml), IL-23 (20 ng/ml) (all Biolegend) added in 100 μl medium, unless otherwise indicated. For TH1 polarization, 10 ng/ml IL-12 and anti-IL-4 (3 μg/ml, both Biolegend) was added. For TREG polarization, culture was in RPMI with TGFβ (10 ng/ml, Biolegend) and IL-2 (10 ng/ml, Peprotech, Rocky Hill, USA). If indicated, 5x these cytokine concentrations were used.
Coating to the cell culture dish was performed for at least 30 min at 37 °C. Coating with purified anti-CD28 (1 μg/ml, clone 37.51) and anti-CD3 leaf (10 μg/ml, clone 17A2) or anti-CD3 ultraleaf (10 μg/ml, clone 145–2C11, all Biolegend) was in a total volume of 7 μl/well. For soluble antibody addition, 1 μg/ml anti-CD28 and 10 μg/ml anti-CD3 were added with the medium. Leaf-IgG anti-CD3 isotype (Biolegend) was given precoated or soluble at a concentration of 10 μg/ml. If indicated, collagen (2 μl, 0.1% stock solution) and RGD (2 μl, 10 mg/ml stock solution) (both Sigma-Aldrich, St. Louis, MI, USA), FCS (10 μl, 10%, in sterile pyrogen free PBS (Lonza, Basel, Switzerland)), bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MI) dissolved in sterile PBS at a concentration of 10 ng/ μl and 0.1% = 10 μg/ μl and filtered (0.2 μm) as indicated, LPS at the indicated concentrations (500000 EU/mg, Escherichia coli O111:B4, Sigma-Aldrich), trypsin (to reach a protease:protein ratio of 1:5–1:20 (w/w) in PBS, Serva Electrophoresis, Heidelberg, Germany), CFA (1 μl of 10% and 1% V/V in PBS as indicated, Sigma-Aldrich) or Montanide ISA 51VG (1 μl of 10% and 1% V/V in PBS as indicated, Elaiapharm, Paris, France) was added to the coating step or in solution. CH-223191 (5 mg/ml in DMSO) and FICZ (1 mg/ml in DMSO, both Sigma-Aldrich) were further diluted in PBS and used in the indicated amount51. Final DMSO concentrations ranged from 1:500 to 1:1,000,000, a range that did not significantly affect the TH17 polarization in our setting (Suppl. Fig. 8). CX3CL1 (1 μg/ml, 1.7 μl or 8.5 μl for final concentrations of 20 and 100 nM, Peprotech and R&D Systems (Minneapolis, MN, USA)), and 5 μl IL-17A (1 μg/ml to reach a final concentration of 50 ng/ml, Miltenyi and R&D Systems) were used pre-coated or in the same amount applied together with medium as indicated above. Coated and soluble cytokine and antibody addition is also detailed in Suppl. Fig. 6.
Cell culture supernatants from CD4+ and CD4− enriched cells were harvested on day 3 without restimulation and added at 1:1 with fresh medium and soluble TH17 polarizing cytokines at standard concentration to splenocytes for 4 days cell culture. If indicated, anti-IL-22 or isotype (polyclonal Goat IgG, final concentration: 2.5μg/ml, R&D Systems, Minneapolis, MN, USA) was applied. CFSE (Life technologies, Darmstadt, Germany) was used according to the manufacturer’s instructions. Re-stimulation before intracellular cytokine staining was with 10 ng/ml PMA and 500 ng/ml ionomycin (both from Sigma-Aldrich) as described39.
Flow cytometry
The following antibodies were used: TCRβ (H57-597), FoxP3 (150D), IL-17A (TC11-18H10.1), IFN-γ (XMG1.2), CD44 (IM7), CD62l (MEL-14), CD69 (H1.2F3) (Biolegend and eBioscience, San Diego, CA, USA). Near-infrared LIVE/DEAD® Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA), Foxp3/Transcription Factor Staining Buffer set (eBioscience) and Fixation/Permeabilization Solution Kit (BD Biosciences) were used according to the manufacturer’s instructions. Flow cytometry analysis was performed on a Becton-Dickinson FACS Canto. Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Gating was performed for live, TCRβ+ cells before analysis of IL-17A, IFN-γ and FoxP3 expression as % of parent.
RNA isolation and real time PCR
RNA was isolated using NucleoSpin® RNAII or NucleoSpin® RNA Plus Kit (Macherey-Nagel, Duren, Germany) and RNA yield and purity determined with a Colibri Microvolume Spectrometer (Titertek-Berthold, Pforzheim, Germany). After reverse transcription (M-MLV-RT, Promega, Mannheim, Germany), real-time PCR was performed on a LightCycler®480 using SYBR-Green (FastStart Taq DNA Polymerase dNTPack, Roche, Grenzach-Wyhlen, Germany). Primers were as follows (5′-3′): HPRT: FP: CAGTCCCAGCGTCGTGATTA, RP: AGCAAGTCTTTCAGTCCTGTC, Il4: FP: GGTCTCAACCCCCAGCTAGT RP: GCCGATGATCTCTCTCAAGTGAT, Il5: FP: CTCTGTTGACAAGCAATGAGACG RP: TCTTCAGTATGTCTAGCCCCTG, Il10: FP: GCTCTTACTGACTGGCATGAG RP: CGCAGCTCTAGGAGCATGTG, Il17a: FP: TTTAACTCCCTTGGCGCAAAA, RP: CTTTCCCTCCGCATTGACAC, Il17f: FP: TGCTACTGTTGATGTTGGGAC, RP: AATGCCCTGGTTTTGGTTGAA, Il21: FP: GGGGACAGTGGCCCATAAATC, RP: GTGCCCCTTTACATCTTGTGG, Il22: FP: ATGAGTTTTTCCCTTATGGGGAC, RP: GCTGGAAGTTGGACACCTCAA, Il22r: FP: ATGAAGACACTACTGACCATCCT, RP: CAGCCACTTTCTCTCTCCGT, Rorgt: FP: CCGCTGAGAGGGCTTCAC, RP: TGCAGGAGTAGGCCACATTAC, Tbet: FP: CAACAACCCCTTTGCCAAAG RP: TCCCCCAAGCAGTTGACAGT, Foxp3: FP: ACTGGGGTCTTCTCCCTCAA RP: CGTGGGAAGGTGCAGAGTAG, Gata3: FP: CTCGGCCATTCGTACATGGAA RP: GGATACCTCTGCACCGTAGC. Data were analyzed with HPRT as a reference gene using LinRegPCR software58.
Proteome analysis of recombinant murine IL-17A preparations
Proteins were separated by SDS-PAGE, gel pieces were destained, dehydrated and digested with 5 ng/μl trypsin (37 °C, 300rpm) after rehydration. Extracted peptides were dried via vacuum centrifugation and separated using a nanoflow reversed phase chromatography system (RSLC, Thermo Fisher Scientific, Germany). For shotgun analysis, peptides enriched on the trap column were eluted with a multistep linear gradient connected to the nano electrospray source of an LTQ orbitrap velos (Thermo Fisher Scientific, Germany) for shotgun or a 4000 Qtrap (AB Sciex, Germany) mass spectrometer. After ionisation using a metal-coated fused silica emitter, most intensive ions according to overview scans were submitted to CID fragmentation. MS data were processed with MaxQuant software (Version 1.2.0.18). MS-spectra were searched in the SwissProt/Uniprot database with a false discovery rate of 0.01 at protein and peptide level as described59.
Statistical analysis
Two-tailed student t-test was used to compare two conditions. If more than two conditions were compared, Bonferroni’s test of selected conditions was applied after ANOVA. P-values <0.05 were considered significant. Data are expressed as mean ± SEM. P values are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Additional Information
How to cite this article: Dong, L. et al. Surface-bound bovine serum albumin carrier protein as present in recombinant cytokine preparations amplifies T helper 17 cell polarization. Sci. Rep. 6, 36598; doi: 10.1038/srep36598 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Basu, R., Hatton, R. D. & Weaver, C. T. The Th17 family: flexibility follows function. Immunol Rev 252, 89–103, doi: 10.1111/imr.12035 (2013).
Muranski, P. & Restifo, N. P. Essentials of Th17 cell commitment and plasticity. Blood 121, 2402–2414, doi: 10.1182/blood-2012-09-378653 (2013).
Zuniga, L. A., Jain, R., Haines, C. & Cua, D. J. Th17 cell development: from the cradle to the grave. Immunol Rev 252, 78–88, doi: 10.1111/imr.12036 (2013).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13, 227–242, doi: 10.1038/nri3405 (2013).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201, 233–240, doi: 10.1084/jem.20041257 (2005).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238, doi: 10.1038/nature04753 (2006).
Mangan, P. R. et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234, doi: 10.1038/nature04754 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo of IL-17-producing T cells. Immunity 24, 179–189, doi: 10.1016/j.immuni.2006.01.001 (2006).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303, doi: 10.1016/j.cell.2012.09.016 (2012).
Zhou, L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8, 967–974, doi: 10.1038/ni1488 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483, doi: 10.1038/nature05969 (2007).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487, doi: 10.1038/nature05970 (2007).
Nikoopour, E., Bellemore, S. M. & Singh, B. IL-22, cell regeneration and autoimmunity. Cytokine 74, 35–42, doi: 10.1016/j.cyto.2014.09.007 (2015).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33, 747–785, doi: 10.1146/annurev-immunol-032414-112123 (2015).
Perusina Lanfranca, M., Lin, Y., Fang, J., Zou, W. & Frankel, T. Biological and pathological activities of interleukin-22. Journal of molecular medicine, doi: 10.1007/s00109-016-1391-6 (2016).
Veldhoen, M., Hirota, K., Christensen, J., O’Garra, A. & Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med 206, 43–49, doi: 10.1084/jem.20081438 (2009).
Ilchmann, A. et al. Impact of culture medium on maturation of bone marrow-derived murine dendritic cells via the aryl hydrocarbon receptor. Mol Immunol 51, 42–50, doi: 10.1016/j.molimm.2012.02.005 (2012).
Kimura, A., Naka, T., Nohara, K., Fujii-Kuriyama, Y. & Kishimoto, T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA 105, 9721–9726, doi: 10.1073/pnas.0804231105 (2008).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109, doi: 10.1038/nature06881 (2008).
Quintana, F. J. et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71, doi: 10.1038/nature06880 (2008).
Peters, M., Dudziak, K., Stiehm, M. & Bufe, A. T-cell polarization depends on concentration of the danger signal used to activate dendritic cells. Immunol Cell Biol 88, 537–544, doi: 10.1038/icb.2010.3 (2010).
Park, J. H., Jeong, S. Y., Choi, A. J. & Kim, S. J. Lipopolysaccharide directly stimulates Th17 differentiation in vitro modulating phosphorylation of RelB and NF-kappaB1. Immunol Lett 165, 10–19, doi: 10.1016/j.imlet.2015.03.003 (2015).
Kim, S. R. et al. Blockade of Interplay between IL-17A and Endoplasmic Reticulum Stress Attenuates LPS-Induced Lung Injury. Theranostics 5, 1343–1362, doi: 10.7150/thno.11685 (2015).
Reynolds, J. M., Martinez, G. J., Chung, Y. & Dong, C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA 109, 13064–13069, doi: 10.1073/pnas.1120585109 (2012).
Guy, B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 5, 505–517, doi: 10.1038/nrmicro1681 (2007).
McKee, A. S. et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol 183, 4403–4414, doi: 10.4049/jimmunol.0900164 (2009).
Lindblad, E. B. Aluminium compounds for use in vaccines. Immunol Cell Biol 82, 497–505, doi: 10.1111/j.0818-9641.2004.01286.x (2004).
Kumar, P., Chen, K. & Kolls, J. K. Th17 cell based vaccines in mucosal immunity. Curr Opin Immunol 25, 373–380, doi: 10.1016/j.coi.2013.03.011 (2013).
Billadeau, D. D., Nolz, J. C. & Gomez, T. S. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol 7, 131–143, doi: 10.1038/nri2021 (2007).
Pociask, D. A. & Kolls, J. K. Integral role of integrins in Th17 development. J Clin Invest 120, 4185–4187, doi: 10.1172/JCI45450 (2010).
Acharya, M. et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest 120, 4445–4452, doi: 10.1172/JCI43796 (2010).
Bazan, J. F. et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644, doi: 10.1038/385640a0 (1997).
Ali, M. T. et al. A novel CX3CR1 antagonist eluting stent reduces stenosis by targeting inflammation. Biomaterials 69, 22–29, doi: 10.1016/j.biomaterials.2015.07.059 (2015).
Jacquelin, S. et al. CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice. Blood 122, 674–683, doi: 10.1182/blood-2013-01-480749 (2013).
Ollivier, V. et al. Fractalkine/CX3CL1 production by human aortic smooth muscle cells impairs monocyte procoagulant and inflammatory responses. Cytokine 21, 303–311 (2003).
Foussat, A. et al. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol 30, 87–97, doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7 (2000).
Fraticelli, P. et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest 107, 1173–1181, doi: 10.1172/JCI11517 (2001).
Staumont-Salle, D. et al. CX(3)CL1 (fractalkine) and its receptor CX(3)CR1 regulate atopic dermatitis by controlling effector T cell retention in inflamed skin. J Exp Med 211, 1185–1196, doi: 10.1084/jem.20121350 (2014).
Dong, L. et al. T Cell CX3CR1 Mediates Excess Atherosclerotic Inflammation in Renal Impairment. J Am Soc Nephrol, doi: 10.1681/ASN.2015050540 (2015).
Nikoopour, E. et al. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. J Immunol 184, 4779–4788, doi: 10.4049/jimmunol.0902822 (2010).
Tigno-Aranjuez, J. T., Jaini, R., Tuohy, V. K., Lehmann, P. V. & Tary-Lehmann, M. Encephalitogenicity of complete Freund’s adjuvant relative to CpG is linked to induction of Th17 cells. J Immunol 183, 5654–5661, doi: 10.4049/jimmunol.0900645 (2009).
Chong, A. S. et al. Reversal of diabetes in non-obese diabetic mice without spleen cell-derived beta cell regeneration. Science 311, 1774–1775, doi: 10.1126/science.1123510 (2006).
Aucouturier, J., Dupuis, L., Deville, S., Ascarateil, S. & Ganne, V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines 1, 111–118, doi: 10.1586/14760584.1.1.111 (2002).
Valitutti, S., Muller, S., Salio, M. & Lanzavecchia, A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med 185, 1859–1864 (1997).
Guy, C. S. et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol 14, 262–270, doi: 10.1038/ni.2538 (2013).
Hayashida, K. et al. Bone marrow changes in adjuvant-induced and collagen-induced arthritis. Interleukin-1 and interleukin-6 activity and abnormal myelopoiesis. Arthritis Rheum 35, 241–245 (1992).
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240, doi: 10.1038/nature06878 (2008).
Ichiyama, K. et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem 283, 17003–17008, doi: 10.1074/jbc.M801286200 (2008).
Jasenosky, L. D., Scriba, T. J., Hanekom, W. A. & Goldfeld, A. E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev 264, 74–87, doi: 10.1111/imr.12274 (2015).
Ndure, J. & Flanagan, K. L. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front Microbiol 5, 477, doi: 10.3389/fmicb.2014.00477 (2014).
Rutz, S. et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol 12, 1238–1245, doi: 10.1038/ni.2134 (2011).
El Malki, K. et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J Invest Dermatol 133, 441–451, doi: 10.1038/jid.2012.318 (2013).
Sonnenberg, G. F. et al. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 207, 1293–1305, doi: 10.1084/jem.20092054 (2010).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203, 2271–2279, doi: 10.1084/jem.20061308 (2006).
Broadhurst, M. J. et al. IL-22 + CD4 + T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2, 60ra88, doi: 10.1126/scitranslmed.3001500 (2010).
Zhao, J. et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 59, 1331–1342, doi: 10.1002/hep.26916 (2014).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Ruijter, J. M. et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic acids research 37, e45, doi: 10.1093/nar/gkp045 (2009).
Schroder, A., Rohrbeck, A., Just, I. & Pich, A. Proteome Alterations of Hippocampal Cells Caused by Clostridium botulinum C3 Exoenzyme. Journal of proteome research 14, 4721–4733, doi: 10.1021/acs.jproteome.5b00591 (2015).
Acknowledgements
S.v.V. was supported by Else Kroener Fresenius Stiftung.
Author information
Authors and Affiliations
Contributions
L.D., A.H., A.W., A.P., H.H. and S.v.V. designed research, L.D., A.H. and A.P. performed experiments, L.D., A.H., A.P. and S.v.V. analyzed data, L.D. and S.v.V. wrote the manuscript with help from all co-authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dong, L., Helmke, A., Waisman, A. et al. Surface-bound bovine serum albumin carrier protein as present in recombinant cytokine preparations amplifies T helper 17 cell polarization. Sci Rep 6, 36598 (2016). https://doi.org/10.1038/srep36598
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36598
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.