Abstract
While disruption of the circadian clock triggers a spectrum of affective abnormalities, how the clock regulates mammalian emotionality remains unclear. Here, we characterized the time-of-day-dependent regulation of mouse anxiety-like behaviors. We show that anxiety-like behaviors are expressed in a circadian manner in mice and demonstrate that the clock machineries in the dorsal telencephalon (dTel) are required for the time-of-day-dependent regulation of anxiety-like behaviors. We identify suprachiasmatic nucleus circadian oscillatory protein (SCOP/PHLPP1β) as an essential intracellular signaling molecule mediating this temporal regulation downstream of the clock. Using viral-mediated, basolateral amygdala (BLA)-specific knockout of Scop, we demonstrate that deletion of SCOP in the BLA exerts anxiolytic effects on the elevated plus maze at early subjective night, thereby blunting the circadian variation in the anxiety-like behavior. We conclude that the circadian expression of SCOP in the BLA plays a key role in generating circadian rhythmicity in the anxiety-like behavior. Our results demonstrate SCOP as a regulator of anxiety-like behaviors and reveal its key roles in the anxiogenic functions of the BLA.
Similar content being viewed by others
Introduction
Approach and avoidance behavior constitutes a core component of animal decision-making, where, in mammals, emotionality shapes the animals’ behavioral tendencies to approach or avoid by modulating appetitive-aversive motivation1,2,3,4. Fear or anxiety, for example, elicits a set of defensive behavioral responses toward an imminent or anticipatory aversive stimulus, respectively5. Recent studies utilizing optogenetic tools have identified various important brain regions and circuitries underlying fear/anxiety responses5. The amygdala is thought to be central to the regulation of fear/anxiety, and activation of basolateral amygdala (BLA) projections to the centromedial amygdala (CeA) elicits anxiolytic responses, whereas somatic activation of BLA neurons is anxiogenic6. Nevertheless, how the regulatory machineries converge to effectuate emotional modulation of behavior remains largely unknown. Molecular mechanisms underlying anxiety regulation are much less well understood, and currently available anxiolytic medications produce their effects through pharmacological mechanisms of action yet to be fully understood7. In order to better understand mammalian anxiety, addressing its regulation from an alternative perspective is of importance.
In light of this, recent findings on the functional relationship between the circadian clock and emotionality-related behaviors could provide an important clue. The circadian clock is an organism’s internal pacemaker system with an intrinsic period of circa 24 hours, where the “master” clock in the hypothalamic suprachiasmatic nucleus (SCN) receives input from retinal photoreceptors and accordingly synchronizes peripheral clocks distributed throughout the body, driving diverse physiological phenomena. Dysfunctions of the circadian clock such as those arising from shift work or jet lag have been linked to a variety of mood disorders8. Conversely, abnormalities in the circadian rhythmicity of various physiological measures have been observed in patients diagnosed with major mood/anxiety disorders9,10. In rodents, perturbations of the circadian clock by means of surgical, genetic, pharmacological, light-induced, or behavioral manipulations lead to a spectrum of abnormalities in emotionality-related behaviors, including elevated or attenuated anxiety-like behaviors11.
Recent evidence points to a mechanism by which dysfunctions in the circadian clockwork lead to abnormal emotionality through aberrant dopaminergic activity in the ventral tegmental area (VTA), a major dopaminergic nucleus12,13. Despite the established roles of dopamine and other monoamine systems in anxiety regulation, their causality in mood/anxiety disorders and sufficiency in the regulation of emotionality per se have been questioned14,15,16,17. Furthermore, while these studies provide important insights into affective abnormalities arising from clock dysfunction, much remains unknown as to how the circadian clock maintains emotionality-related behaviors at physiological levels. In humans, both positive and negative affect are reported to display diurnal variation18, whereas excessive diurnal variations in mood states are a hallmark of major depressive and bipolar disorders19, implicating the physiological importance of precise time-of-day-dependent regulation of emotionality.
In the present study, we sought to unravel the mechanisms governing mammalian anxiety regulation and characterized temporal regulation of mouse anxiety-like behaviors by the circadian clock. We examined the involvement of SCOP (SCN circadian oscillatory protein), a signaling molecule originally identified as a gene product whose expression oscillates in a circadian manner in the rat SCN20. SCOP is a 183-kDa protein comprising pleckstrin homology (PH), leucine-rich repeat, protein-phosphatase 2C-like, glutamine-rich, and PDZ-binding domains, and SCOP has been shown to regulate a range of intracellular signaling pathways21,22,23. In the mouse hippocampus, SCOP plays an essential role in the consolidation of long-term object recognition memory24. Here, we describe SCOP-mediated time-of-day-dependent regulation of anxiety-like behaviors.
Results
Anxiety-like behaviors in wild-type mice are under circadian regulation
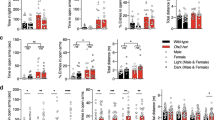
In order to examine the temporal regulation of anxiety by the circadian clock, we profiled time-of-day-dependent variations in anxiety-like behaviors of wild-type (WT) mice. To evaluate mouse anxiety-like behaviors, we utilized the elevated plus maze (EPM) and open field (OF) tests. These paradigms are based on rodents’ intrinsic conflict between the drive to explore novel environments and the tendency to avoid open space; thus, increased time spent in the open arms of the EPM or in the center area of the OF is thought to represent reduced anxiety25,26. One group of mice (“LD” group, n = 24) were tested under a light-dark (LD) cycle at one of four or eight zeitgeber times (ZTs; lights on at ZT0, off at ZT12). The other group (“dLL” group, n = 76) were housed under a constant dim light condition (dLL) for >24 hrs and tested on the second day under dLL at one of four or eight projected circadian times (CTs; subjective day starts at CT0 and ends at CT12) (Fig. 1a). This enables us to eliminate the effects of cyclic light conditions. Testing CTs were projected using a circadian period (τ) of 25.0 hrs, as our mouse strains consistently exhibited free-running activity rhythms with a τ of 25.05 ± 0.07 hr SEM (n = 6). All mice were handled daily for >1 week prior to behavioral assays for acclimation. Housing under dLL had no observable effects on sleep/wake cycles or general activity rhythms.
Diurnal and circadian expression of anxiety-like behaviors in wild-type mice on elevated plus maze (EPM, (b–d)) and open field (OF, (e,f)) tests. (a) Timeline for behavioral assays. LD: Mice were kept under an LD cycle and tested at one of 4 or 8 ZTs (Zeitgeber Time). dLL: Mice were placed under constant dim light 1 day prior to the testing and assayed at one of 4 or 8 CTs (Circadian Time). (b–d), Entries into open arms ((b), ratio of open:total entries), the time spent on open arms (c), and the total number of entries (d) for EPM. (e,f) The time spent in the center area (e) and the total distance traveled (f) for OF under LD (black) and dLL (orange) conditions. #P < 0.05 between ZTs/CTs by one-way ANOVA. n = 6 per data point for EPM LD and OF LD, n = 12 per data point for EPM dLL, n = 7–12 for OF dLL.
In both the EPM and OF tests, anxiety-like behaviors exhibited diurnal variation and circadian rhythmicity under LD and dLL, respectively (Fig. 1b–f). Anxiety-like behaviors in the EPM and OF tests (hereafter referred to as “EPM-anxiety-like” and “OF-anxiety-like” behaviors, respectively) showed rhythms almost anti-phasic to each other: EPM-anxiety-like behavior was high at early subjective night (active phase) and low at early subjective day (resting phase), while OF-anxiety-like behavior was high at early subjective day and low at early subjective night (Fig. 1b,c,e). These results support the notion that EPM- and OF-anxiety-like behaviors reflect distinct physiological phenomena25,26,27 (See Discussion). No significant variation in the numbers of total arm entries or in general locomotor activity levels was found across the day except for OF total distance under LD (Fig. 1d,f), rendering it unlikely that circadian changes in general activity levels play a major role in the circadian expression of anxiety-like behaviors. The diurnal profiles were similar between the LD and dLL groups, suggesting that mouse anxiety-like behaviors are dynamically regulated by the intrinsic circadian clock rather than by external light conditions.
The circadian clock in the dorsal telencephalon drives rhythmic anxiety-like behaviors
To examine the regulation of anxiety-like behaviors by the circadian clock, we used Bmal1fl/fl Emx1Cre/+ conditional knockout (cKO) mice. Emx1Cre expression is restricted to glutamatergic neurons and astrocytes in the dorsal telencephalon (dTel), which includes the neocortex, hippocampus, and BLA28. Bmal1fl/fl Emx1Cre/+ mice lack BMAL1 protein in the dTel29. BMAL1 and CLOCK, both bHLH transcription factors, heterodimerize to activate E-box-mediated transcription and hence constitute the core transcriptional-translational feedback loop of the molecular circadian clock. Cells lacking BMAL1 lose circadian rhythmicity30. This cKO enables us to eliminate the effects from clock dysfunctions in the SCN, which leads to a systemic loss of circadian rhythmicity, and in the monoamine-producing nuclei in the midbrain. We first examined the effects of Bmal1 cKO on the circadian expression of clock genes in amygdala subnuclei: BLA, a dTel subnucleus involved in the regulation of anxiety-like behaviors5,6, and CeA, a ventral telencephalic subnucleus. Both in the BLA and CeA of littermate WT mice, mRNA levels of Bmal1, Dbp, and Rev-erbα exhibited circadian variations (Fig. 2a–c). In the BLA of Bmal1 cKO mice, Bmal1 mRNA levels were downregulated by >3 fold, and Dbp and Rev-erbα expression was constantly low across the day (Fig. 2a–c, blue). Clock gene expression in the CeA was unaffected by Bmal1 cKO (Fig. 2a–c, red), consistent with the lack of Emx1 expression in the CeA28.
Circadian machineries in the dorsal telencephalon (dTel) regulate anxiety-like behaviors.
(a–c) Circadian mRNA expression of clock genes Bmal1 (a) Dbp (c) and Rev-erbα/Nr1d1 (C) in the BLA (blue lines, circles) and CeA (red lines, triangles) of Bmal1 cKO mice (“cKO”, Bmal1fl/fl Emx1Cre/+) (solid lines) and littermate wild-type mice (“WT”, Bmal1fl/fl Emx1+/+) (dashed lines) at CTs 2, 8, 14, 20. Data are normalized to Rps29. (d) % Open entries, (e) time spent on open arms, (f) Numbers of total arm entries in the EPM test for cKO and WT mice; (g) Center time and H, total distance traveled in the OF test for cKO and WT mice at CT2 (shaded bars) and CT14 (filled bars). (a–c) #P < 0.05 among CTs by one-way ANOVA, *p < 0.05 vs WT at the corresponding CT. n = 3 per data point. (d–h) *p < 0.05 by unpaired Student’s t-test. n for WT: CT2, 9; CT14, 10. n for cKO: CT2, 8; CT14, 8. Data are means with SEM.
Bmal1 cKO mice exhibited normal activity rhythms (Supplementary Fig. 1) and appeared physically normal. Whereas littermate WT mice (Bmal1fl/fl Emx1+/+) reproduced the circadian variations in anxiety-like behaviors of WT mice (Fig. 1) at CT2 (early subjective day) and CT14 (early subjective night), anxiety-like behaviors remained high in Bmal1 cKO mice both during the day and night at levels comparable to the peak levels in littermate WT mice (Fig. 2d–h). Circadian variations in anxiety-like behaviors between CT2 and CT14 were not observed in Bmal1 cKO mice (Fig. 2d,e,g). Bmal1 cKO had no significant effect on general locomotor activities (Fig. 2f,h).
SCOP is expressed in a circadian manner in the amygdala
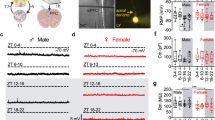
Given the involvement of the circadian clock in the temporal regulation of anxiety-like behaviors, we examined the roles of SCOP, a clock-controlled signaling protein. We first examined the expression of SCOP protein in dTel subregions. The prefrontal cortex, hippocampus, and BLA appeared to express SCOP in a circadian manner with higher expression at night (Supplementary Fig. 2). We then narrowed our focus on the BLA, a region known to play major roles in anxiety regulation5,6, and more fully profiled SCOP expression. Both mRNA and protein levels of SCOP exhibited circadian variations in the BLA, with mRNA peaking at CT8 and protein peaking at CT14 (Fig. 3a–c, blue). SCOP was also rhythmically expressed in the CeA, exhibiting a profile anti-phasic to that in the BLA (Fig. 3a–c, red), consistent with an earlier study reporting anti-phasic diurnal expression of the clock component PERIOD2 between the BLA and CeA31. In the BLA of Bmal1 cKO mice, Scop mRNA levels were constantly high across the day with no significant circadian rhythmicity (Fig. 3d). Combined with the constantly low expression of E-box-regulated clock genes Dbp and Rev-erbα across the day in the BLA of Bmal1 cKO mice (Fig. 2g,h), these results suggest indirect Bmal1-mediated transcriptional repression of Scop presumably through REV-ERB-mediated repression at the REV-ERB binding sites found in the intron 1 of Scop gene32, a genomic region conserved among placental mammals.
SCOP is rhythmically expressed in the amygdala.
(a–c) Levels of Scop mRNA ((a) quantitative PCR) and SCOP protein ((b,c) immunoblotting) in the BLA (blue) and CeA (red) at CTs 2, 8, 14, 20. Representative immunoblots against SCOP and β-actin (b) and quantification of all samples (c) are shown. (d) Scop mRNA levels in the BLA (blue lines, circles) and CeA (red lines, triangles) of Bmal1 cKO (solid lines) and littermate WT (dashed lines) mice, normalized to Rps29.#P < 0.05 between CTs by one-way ANOVA. n = 3 per data point. Data are means with SEM.
SCOP in the dorsal telencephalon is required for circadian expression of anxiety-like behaviors
To examine the involvement of SCOP in anxiety regulation, we used dTel-specific Scop cKO mice (Scopfl/fl Emx1Cre/+)29. In the BLA, Scop mRNA levels were undetectable both at CT8 and CT20 in Scop cKO mice (Fig. 4a, blue), whereas Scop expression in the CeA was not significantly affected (Fig. 4a, red). Scop cKO mice exhibited no observable defects in circadian activity rhythms or sleep/wake cycles29 or in general locomotor activity (Fig. 4d,f). Circadian expression of Bmal1 was intact in the BLA and CeA of Scop cKO mice (Supplementary Fig. 3). In both the EPM and OF tests, Scop cKO mice failed to express circadian changes in anxiety-like behaviors: While littermate WT mice (Scopfl/fl Emx1+/+) reproduced the circadian variations in anxiety-like behaviors of WT mice (Fig. 1) between CT2 (day) and CT14 (night), anxiety-like behaviors remained constantly low at both CTs in Scop cKO mice (Fig. 4b–f), a phenotype distinct from that of Bmal1 cKO mice (Fig. 2). This is in line with the elevated Scop expression observed in the BLA of Bmal1 cKO mice (Fig. 3d), leading us to hypothesize that changes in SCOP levels in the BLA have direct effects on anxiety-like behaviors.
SCOP in the dTel is required for circadian expression anxiety-like behaviors at CTs 2 and 14.
(a) Scop mRNA levels in the BLA (blue) and CeA (red) of cKO and WT mice at CT8 (light color) and CT20 (dark color). n.d., not detected. (b) % Open entries (c) time spent on open arms (d) Numbers of total arm entries for Scop cKO mice (“cKO”, Scopfl/fl Emx1Cre/+) and littermate WT mice (“WT”, Scopfl/fl Emx1+/+). (e) Center time and (f) total distance traveled for cKO and WT mice. *p < 0.05 by unpaired Student’s t-test n.d.: not detected. (a–e) n for WT: CT2, 14; CT14, 13. n for cKO: CT2, 9; CT14, 9. (f) n = 3 per data point. Data are means with SEM.
SCOP in the BLA has an anxiogenic function and is essential for circadian expression of anxiety-like behavior in the EPM test
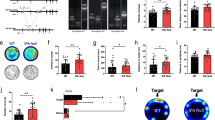
To investigate the function of SCOP in the BLA, we examined the effects of Scop knockout (KO) in the BLA. To this end, we constructed adeno-associated virus (AAV) expressing Cre recombinase fused to EGFP (“AAV-Cre”) or EGFP alone (“AAV-GFP”) driven under human synapsin (hSyn) promoter (Fig. 5a). In vitro analyses using cultured cells confirmed the recombinase activity of the fusion protein, Cre expression in neuronal cells, and the infectious ability of the viral constructs in neurons (Supplementary Fig. 4). We then injected AAV-Cre or AAV-GFP bilaterally into the BLA of Scopfl/fl mice (Scop BLA KO) and examined the effects of BLA-specific Scop KO on anxiety-like behaviors (Fig. 5b). Transduction with AAV-Cre resulted in a >3 fold reduction in Scop mRNA levels in the BLA of Scopfl/fl mice compared to the BLA of Scopfl/fl mice transduced with AAV-GFP (Fig. 5c–e). Behaviorally, -BLA-specific Scop KO mice phenocopied Scop cKO mice in the EPM test, exhibiting lower anxiety-like behavior both at CT2 and CT14 (Fig. 5f–h). BLA-specific Scop KO abrogated the circadian variation in open arm entries in the EPM test between the CTs (Fig. 5f,g). The effects of BLA-specific Scop KO on open arm entries at CT14 were significant compared to mice bilaterally transduced with AAV-GFP. The total numbers of entries were unaffected (Fig. 5h). AAV-GFP-transduced mice exhibited circadian changes in open arm entries at levels comparable to that in wild-type (Fig. 1b) or Scopfl/fl mice (Fig. 4b). BLA-specific Scop KO had no statistically significant effect on anxiety-like behavior in the OF test (Fig. 5i,j), suggesting region-specific roles of SCOP in regulating anxiety-like behaviors in the EPM and OF tests (See Discussion). These results demonstrate that SCOP in the BLA functions to elevate anxiety-like behavior in the EPM test at early subjective night (CT14), when SCOP level in the BLA peaks.
SCOP in the BLA is required for the circadian changes in EPM-anxiety-like behavior.
(a) AAV vectors expressing iCre::EGFP fusion protein (“AAV-Cre”) or EGFP (“AAV-GFP”) were constructed (See smentary Information). hSyn: human synapsin promoter; WPRE: woodchuck hepatitis virus posttranscriptional regulatory element; pA: poly-A; ITR: inverted terminal repeat. (b) Timeline for the viral injection, behavioral tests (EPM and OF) and histological inspection. (c) Scop and EGFP mRNA levels in the BLA of Scopfl/fl mice injected with AAV-GFP (open bars) or AAV-Cre (filled bars). (d) Histological reconstruction of the injection sites in coronal sections of the mouse brain. Values are distances in millimeters from Bregma (minus indicates a position posterior to Bregma) according to the mouse stereotaxic atlas by Paxinos and Watson47. AAV-Cre (magenta) or AAV-GFP (blue) was injected bilaterally to the BLA (shown in cyan) for each hemisphere of an individual mouse. Each colored circle represents the site of the strongest GFP signal. (e) A representative image of the infection are in the BLA. (f–j) Anxiety-like behavior in BLA-specific Scop KO mice. EPM % Open entries (f), time spent on open arms (g), total numbers of entries into closed and open arms (h), OF center time (i), and OF Total distance (j) at CT2 (shaded bars) and CT14 (filled bars) for mice bilaterally infected with AAV-Cre (“Cre”) and mice bilaterally infected with AAV-GFP (“GFP”). *p < 0.05 by unpaired Student’s t-test. n for behavioral assays: Cre, 6 (CT2) and 8 (CT14) for EPM, 8 (CT2) and 6 (CT14) for OF; GFP, 6 (CT2) and 6 (CT14). Data are means with SEM. Scale bars, 500 μm.
Discussion
Our results demonstrate that the anxiogenic function of SCOP in the BLA drives circadian rhythms in EPM-anxiety-like behavior in mice. We show that the clock machinery and SCOP in the dTel are required for the circadian expression of anxiety-like behaviors in both the EPM and OF tests (Figs 2 and 4). Bmal1 cKO leads to upregulation of SCOP expression in the BLA (Fig. 3d) and has anxiogenic effects, whereas Scop cKO has anxiolytic effects. Deletion of SCOP in the BLA abolishes the diurnal elevation of EPM-anxiety-like behavior at early subjective night (CT14) (Fig. 5f,g), signifying the role of SCOP in the BLA in driving the anxiety-like behavior in the EPM test. Collectively, we conclude that SCOP is a regulator of mouse anxiety-like behaviors functioning in anxiogenesis, driving circadian changes in these behaviors, and that the rhythmic expression of SCOP in the BLA is required for the circadian expression of anxiety-like behavior in the EPM test.
BLA-specific deletion of SCOP resulted in a loss of the circadian variation in anxiety-like behaviors in the EPM test (Fig. 5f,g) but not in the OF test (Fig. 5i,j), while dTel-specific deletion of SCOP abolished the rhythms in both behaviors (Fig. 4b–f). These data suggest that while SCOP in the dTel is required for the circadian variation in anxiety-like behaviors in the OF test, SCOP in the BLA, which is part of the dTel, is not. It is therefore likely that a dTel subregion(s) other than the BLA is responsible for the circadian regulation of anxiety-like behaviors in the OF test.
Our results also demonstrate an anti-phasic relationship in the circadian expression of SCOP in the BLA and CeA (Fig. 3). Antiphasic expression of the clock gene Period2 in these amygdala nuclei have previously been reported31, and our data demonstrate that these nuclei also exhibit distinct phase distributions in the circadian expression of clock genes Bmal1, Rev-erbα, and Dbp (Fig. 2a–c). Previous studies have reported roles of tissue-specific transcriptional co-regulators, such as CBP/p300, in regulating tissue- and cell type-specific circadian transcription and phase distributions33,34. We thus speculate that BLA- and CeA-specific co-activators and/or co-repressors may regulate distinct rhythms in mRNA and protein levels of SCOP and clock genes, although we have been unable to directly examine protein levels of core clock genes in the amygdala subregions potentially due to their low abundance.
SCOP is a clock-controlled multi-domain signaling molecule regulating various intracellular signaling pathways20,21,22,23,24,35. Notably, SCOP and its shorter variant PHLPP1α have been shown to dephosphorylate AKT and thereby suppress PI3K/AKT/GSK3β signaling, where signaling cascades downstream of dopamine D2, 5-HT1A and 5-HT2 receptors converge36. In humans and mice, AKT1, AKT2, and GSK3β have been associated with abnormal anxiety37,38,39. These data point to a model in which SCOP regulates anxiety by modulating AKT/GSK3β signaling. In addition, SCOP has been shown to regulate K-Ras/ERK signaling in the hippocampus24,40. MAPK/ERK signaling in the amygdala has been repeatedly shown to contribute to anxiety regulation in rodents. For example, exposure to novel environments or repeated intraperitoneal injection of corticosterone, which enhances anxiety-like behavior in the EPM test, increases ERK activation in the rat amygdala41,42, whereas ERK inhibition in the mouse BLA abolishes restraint stress-induced increase in anxiety-like behavior in the EPM test43. Given the crosstalk reported for MAPK/ERK and AKT/GSK3β signaling pathways, it is therefore of interest to examine how SCOP regulates these anxiety-related signaling pathways in the BLA in vivo.
Importantly, the anxiogenic and anxiolytic effects of Bmal1 and Scop cKO, respectively, did not exceed the range of the circadian variations of anxiety-like behaviors expressed by wild-type mice (Figs 2 and 4). Put another way, the loss of circadian rhythmicity in the dTel appears to “halt” the circadian expression of anxiety-like behaviors at a specific time of day. We therefore speculate that the circadian clock in the dTel confers circadian rhythmicity on mouse anxiety-like behaviors. This, together with our finding that the anxiety-like behaviors in the EPM and OF tests peak at distinct times of day (Fig. 1b,c,e), suggests that the rhythmic expression of anxiety-like behaviors may play an important survival function. In this regard, examining whether and how this circadian expression is conserved in other species, especially in diurnal animals, will be of particular interest.
It has to be noted that the distinct temporal profiles in the expression of anxiety-like behaviors in the EPM and OF tests are not an unexpected finding, as these behaviors have been frequently suggested to reflect distinct physiological phenomena. For example, factor analysis studies using rodents revealed that anxiety-related behaviors evaluated in the EPM and OF tests do not load on a common factor44,45. Consistently, multiple pharmacological manipulations have been shown to produce test-selective effects on these anxiety-like behaviors46. Hence, a current model considers the anxiety-like behaviors evaluated in the EPM and OF tests, as well as in other anxiety-related tests, as representing partially overlapping but distinct aspects of the multidimensional “anxiety”, likely underlain by partially overlapping mechanisms26. Not only are our results (Fig. 1b,c,e) compatible with such a model, our finding that clock dysfunction in the dTel abolishes the distinct phase relationship between the EPM- and OF-anxiety-like behaviors (Fig. 2) suggests that the circadian clock may be actively involved in the differentiation of these anxiety-like behaviors. Although further study is necessary to elucidate the underlying mechanisms, these results, combined with the selective effects of Scop BLA KO on the EPM but not the OF test (Fig. 5f–j), could provide a foundation for unraveling the complexity of anxiety-like behaviors.
In summary, we have described the circadian expression of mouse anxiety-like behaviors, which requires clock machineries and SCOP function in the dTel, and demonstrated that SCOP in the BLA is expressed in a circadian manner and exerts anxiogenic effects on the EPM. While the molecular mechanisms remain to be established, we conclude that circadian regulation of SCOP levels in the dTel plays an essential role in generating the circadian rhythmicity in anxiety-like behaviors, which could represent an important function in animal survival. By characterizing the involvement of a temporal axis in the regulation of mammalian anxiety-like behavior, as well as by identifying key molecular and neuroanatomical players therein, our present study provides a new foundation for future research on animal emotionality. Further understanding of the intrinsic plasticity in anxiety regulation described here could potentially enable the development of new classes of treatment for anxiety/mood disorders.
Methods
Animals and housing
All experiments were conducted in accordance with guidelines set by The University of Tokyo and approved by the Committee on Animal Care and Use of the Graduate School of Science at The University of Tokyo. Wild-type male C57BL/6J mice were purchased from Tokyo Laboratory Animals Science Co., Ltd (Tokyo, Japan). Emx1Cre/+28 mice were kindly provided by Dr. Atsu Aiba. All animals were initially housed under a 12 hr light/12 hr dark cycle (lights on at 8 am) in temperature- and humidity-controlled compartments with food and water available ad libitum. More information is available in Supplementary Information.
Surgery
Male Scopfl/fl mice aged 8–10 weeks were deeply anesthetized with a mixture of ketamine (140 mg/kg) and xylazine (8.8 mg/kg) in bacteriostatic saline given intraperitoneally (20 ml/kg) and placed on a stereotactic apparatus (Narishige, Tokyo, Japan). The skull was exposed, and holes were drilled bilaterally above the basolateral amygdala. The coordinates relative to bregma were: anteroposterior, −1.65 mm; lateral, +3.30 mm; dorsoventral, −4.45 mm. Mice were bilaterally injected with 0.5 μl of either AAV-Cre or AAV-GFP over 5 min, and the needles were kept in place for an additional 5 min to ensure infusion.
Behavioral assays
Male mice aged 12–16 weeks (wild-type and AAV-injected Scopfl/fl) or 20–25 weeks (Bmal1 and Scop cKO) were subjected to behavioral tests. Littermate Bmal1fl/fl Emx1+/+46 or Scopfl/fl Emx1+/+ mice were used for control. Prior to testing, all mice were singly housed, entrained to the LD cycle for >2 weeks, and handled daily for acclimation at random times of day for >1 week. All behavioral assays were conducted under dim light at 4.0 ± 0.1 lux at the center of the apparatuses. Mice were picked up on the operator’s palms and released into the apparatuses such that the mice voluntarily walk into the maze or open field from the palms. For BLA-specific KO experiments, behavioral data from mice with clear bilateral GFP signal in the basolateral amygdalar complex were adopted as AAV-Cre or AAV-GFP data. For fluorescent microscopy, AAV-injected mice were sacrificed by rapid cervical dislocation, and the brain was removed, mounted on a brain matrix (ASI Instruments), and sliced into 1-mm thick sections. The sections were analyzed for GFP signal under a fluorescent stereoscopic microscope (Leica). Detailed procedures are described in Supplementary Information.
Elevated Plus Maze
Mice were placed in the center of an elevated plus maze (O’Hara & Co., Ltd., Tokyo, Japan) facing one of the closed arms. The maze has four arms (5 × 25 cm), the opposing two of which are protected with clear walls (16 cm high), and is elevated 50 cm from the ground. Mice were allowed to freely explore the maze for 5 min; their behavior was monitored with an automated video tracking system, and the time spent on open arms and entries into open/closed arms were determined using TIME_EP software (O’Hara & Co., Ltd.) or manually by an analyst blinded to time-of-day information (see Data analysis).
Open Field
Mice were placed into one of the corners of a 45 × 45 cm open field (O’Hara & Co., Ltd.), and their behavior was monitored for 5 min with an automated video tracking system. The time spent in the center of the field (30% area, circular) and the distance traveled were determined using ImageJ software (NIH, MD) with OpenField plug-in (O’Hara & Co., Ltd.).
Data analysis
Behavioral analyses were automated except for the elevated plus maze (EPM) test for wild-type C57BL/6J mice, for which all recorded behavioral data were shuffled into random orders, and entries into open and closed arms and the time spent on each arm were recorded by an operator blinded to time-of-day information for each subject. One-way ANOVA tests were used to examine the statistical significance of data consisting of three or more groups. Unpaired two-tail Student’s t-tests were used for analysis on data with two groups. All data are presented as means with SEM. P values are presented as “P” for ANOVA tests and “p” for t-tests.
Additional Information
How to cite this article: Nakano, J. J. et al. SCOP/PHLPP1β in the basolateral amygdala regulates circadian expression of mouse anxiety-like behavior. Sci. Rep. 6, 33500; doi: 10.1038/srep33500 (2016).
References
Hull, C. L. A behavior system; an introduction to behavior theory concerning the individual organism (Yale University Press, 1952).
Tooby, J. & Cosmides, L. The past explains the present: Emotional adaptations and the structure of ancestral environments. Ethol Sociobiol 11, 375–424 (1990).
Adams, R. B. Jr., Ambady, N., Macrae, C. N. & Kleck, R. E. Emotional expressions forecast approach-avoidance behavior. Motiv Emot 30, 177–186 (2006).
Corr, P. J. Approach and avoidance behaviour: Multiple systems and their interactions. Emotion Review 5, 285–290 (2013).
Tovote, P., Fadok, J. P. & Lüthi, A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16, 317–331 (2015).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Farach, F. J. et al. Pharmacological treatment of anxiety disorders: current treatments and future directions. J Anxiety Disord 26, 833–843 (2012).
Scott, A. J. Shift work and health. Prim Care 27, 1057–1079 (2000).
Wulff, K., Gatti, S., Wettstein, J. G. & Foster, R. G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11, 589–599 (2010).
McClung, C. A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther. 114, 222–232 (2007).
McClung, C. A. Circadian rhythms and mood regulation: Insights from pre-clinical models. European Neuropsychopharmacology 21, S683–S693 (2011).
Mukherjee, S. et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol. Psychiatry 68, 503–511 (2010).
Chung, S. et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 157, 858–868 (2014).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Maes, M. et al. (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: From antioxidants to kinase inhibitors. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 659–663 (2011).
Sanacora, G., Treccani, G. & Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77 (2012).
Holmes, A., Heilig, M., Rupniak, N. M. J., Steckler, T. & Griebel, G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci 24, 580–588 (2003).
Golder, S. A. & Macy, M. W. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science 333, 1878–1881 (2011).
Hall, P., Spear, F. G. & Stirland, D. Diurnal variation of subjective mood in depressive states. Psychiatr Q 38, 529–536 (1964).
Shimizu, K., Okada, M., Takano, A. & Nagai, K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett 458, 363–369 (1999).
Shimizu, K., Okada, M., Nagai, K. & Fukada, Y. Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J Biol Chem 278, 14920–14925 (2003).
Jackson, T. C., Verrier, J. D., Semple-Rowland, S., Kumar, A. & Foster, T. C. PHLPP1 splice variants differentially regulate AKT and PKCα signaling in hippocampal neurons: characterization of PHLPP proteins in the adult hippocampus. J Neurochem 115, 941–955 (2010).
Shimizu, K., Mackenzie, S. M. & Storm, D. R. SCOP/PHLPP and its functional role in the brain. Mol Biosyst 6, 38–43 (2010).
Shimizu, K., Phan, T., Mansuy, I. M. & Storm, D. R. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell 128, 1219–1229 (2007).
Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F. & Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134, 49–57 (2002).
Ramos, A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 29, 493–498 (2008).
Ramos, A., Pereira, E., Martins, G. C., Wehrmeister, T. D. & Izídio, G. S. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res 193, 277–288 (2008).
Iwasato, T. et al. Dorsal telencephalon-specific expression of Cre recombinase in PAC transgenic mice. Genesis 38, 130–138 (2004).
Shimizu, K. et al. SCOP/PHLPP1β mediates circadian regulation of long-term recognition memory. Nat. Commun. 7, 12926 10.1038/ncomms12926 (2016).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Lamont, E. W., Robinson, B., Stewart, J. & Amir, S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 102, 4180–4184 (2005).
Cho, H. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012).
Hosoda, H. et al. CBP/p300 is a cell type-specific modulator of CLOCK/BMAL1-mediated transcription. Mol Brain 2, 34 (2009).
Korenčič, A. et al. Timing of circadian genes in mammalian tissues. Sci. Rep. 4, 5782 (2014).
Brognard, J. & Newton, A. C. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab 19, 223–230 (2008).
Beaulieu, J.-M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci 37, 7–16 (2012).
Prickaerts, J. et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci 26, 9022–9029 (2006).
Yang, C. et al. Association between AKT1 gene polymorphisms and depressive symptoms in the Chinese Han population with major depressive disorder. Neural Regen Res 7, 235–239 (2012).
Leibrock, C. et al. Akt2 deficiency is associated with anxiety and depressive behavior in mice. Cell. Physiol. Biochem. 32, 766–777 (2013).
Liu, Y. et al. Deleting both PHLPP1 and CANP1 rescues impairments in long-term potentiation and learning in both single knockout mice. Learning & Memory 23, 399–404 (2016).
Lim, H. et al. Enhancement of Anxiety and Modulation of TH and pERK Expressions in Amygdala by Repeated Injections of Corticosterone. Biomolecules & therapeutics 20, 418–424 (2012).
Sanguedo, F. V. et al. Reciprocal activation/inactivation of ERK in the amygdala and frontal cortex is correlated with the degree of novelty of an open-field environment. Psychopharmacology (Berl.) 233, 841–850 (2016).
Maldonado, N. M., Espejo, P. J., Martijena, I. D. & Molina, V. A. Activation of ERK2 in basolateral amygdala underlies the promoting influence of stress on fear memory and anxiety: influence of midazolam pretreatment. Eur Neuropsychopharmacol 24, 262–270 (2014).
Trullas, R. & Skolnick, P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl.) 111, 323–331 (1993).
Ramos, A., Mellerin, Y., Mormède, P. & Chaouloff, F. A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav Brain Res 96, 195–205 (1998).
Vendruscolo, L. F., Takahashi, R. N., Brüske, G. R. & Ramos, A. Evaluation of the anxiolytic-like effect of NKP608, a NK1-receptor antagonist, in two rat strains that differ in anxiety-related behaviors. Psychopharmacology (Berl.) 170, 287–293 (2003).
Shimba, S. et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 6, e25231 (2011).
Paxinos, G. & Franklin, K. B. J. The mouse brain in stereotaxic coordinates (Academic Press, 2001).
Acknowledgements
This work was supported by KAKENHI Grants-in-Aid for Scientific Research (Grant numbers 24227001 to Y.F. and 15K12767 to K.S.) from Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Drs Atsu Aiba, Takahiko Matsuda, and Andras Nagy for the generous gifts of Emx1Cre/+ mice, pCAG-iCre plasmid, and pCCALL2 plasmid, respectively.
Author information
Authors and Affiliations
Contributions
J.J.N., K.S. and Y.F. designed the research, analyzed data, and wrote the main manuscript. J.J.N. performed the research. S.S. provided Bmal1fl/fl mouse. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nakano, J., Shimizu, K., Shimba, S. et al. SCOP/PHLPP1β in the basolateral amygdala regulates circadian expression of mouse anxiety-like behavior. Sci Rep 6, 33500 (2016). https://doi.org/10.1038/srep33500
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33500
This article is cited by
-
Diurnal variation in declarative memory and the involvement of SCOP in cognitive functions in nonhuman primates
Molecular Brain (2023)
-
The circadian clock in the piriform cortex intrinsically tunes daily changes of odor-evoked neural activity
Communications Biology (2023)
-
Influence of diurnal phase on behavioral tests of sensorimotor performance, anxiety, learning and memory in mice
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.