Abstract
Layered material MoS2 has been attracting much attention due to its excellent electronical properties and catalytic property. Here we report the synthesis of vertically standing MoS2 triangles on silicon carbon(SiC), through a rapid sulfidation process. Such edge-terminated films are metastable structures of MoS2, which may find applications in FinFETs and catalytic reactions. We have confirmed the catalytic property in a hydrogen evolution reaction(HER). The Tafel slope is about 54mV/decade.
Similar content being viewed by others
Introduction
In the past few years, transition metal dichalcogenides(TMD) have attracted great attention for their considerable potential applications in the fields of catalysis, microeletronics, optoelectronic devices on both conventional and flexible substrates1,2,3,4,5,6,7,8,9. Lots of efforts have been made to realize the applications of TMD. But almost all of these works that have been done are based on the platelet-like morphology on the surface of different substrates. In contrast, a few works have been done to use the edges of these layered materials. In fact, vertically standing TMD materials hold high aspect ratio and dangling bonds10. For MoS2, the exposed edges means the S dangling bonds. It is the active sites for catalytic reactions. As a layered material, MoS2 usually expose the basal planes (it means the flat triangles with the Mo atom terminating surface) as the terminating surface with minimal roughness and dangling bonds. But in the condition of vertically standing MoS2, the edges sites were exposed maximally. The exposed edges with high chemical active and may play an important role in many catalytic reactions, such as hydrogen production11,12,13,14,15, photocatalysis16, hydrogen evolution reaction(HER), hydrodesulfurization catalyst used for removing sulfur compounds from oil17,18,19,20,21,22,23. In addition, these vertical structures of TMD are ideal channel materials for FinFET24. However, edges are usually the rare surface sites of layered materials due to their inherently high surface energy. Increasing the edge dimension is therefore challenging.
Methods
In this work, we develop a rapid sulfidation process through a large carrier gas flow rate on SiC using a CVD method. The MoS2 triangles are aligned vertically to the surface of the substrate. Various characterizations techniques were used to have a good understand to the mechanism of the vertically standing triangles. Furthermore, HER performance of vertically standing MoS2 triangles was researched.
The synthesis process of vertically standing MoS2 triangles is schematically illustrated in Fig. 1. At the beginning, MoO3 powder was placed in the centre of the furnace, 6H SiC was placed next to the MoO3 powder. Sulphur powder was placed inside of a steel cylinder out side of the furnace, with a heating tape around it. High pure argon was chosen as carrier gas to convey sulphur and MoO3 vapor to downstream. The temperature of MoO3 powder was 1000 °C, sulphur powder was heated to 260 °C, the growth pressure was atmospheric.
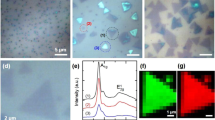
A typical optical image of the MoS2 triangles grown on SiC substrate is shown in Fig. 2a. It clearly shows that most of the triangles on the surface were vertically standing, there are a few flat triangles on the surface. The SEM image demonstrates that the as grown triangles are nearly perpendicular to the substrate. There is a small angles of inclination of some triangles. The edges of different MoS2 triangles could be clearly observed in SEM images. The lateral dimensions of the triangles are tens micrometer, the heights are from 30 nm to 2 μm. Figure 2c is the Raman spectrum of the vertically standing triangles on SiC and monolayer MoS2 on sapphire. Two Raman characteristic bands of vertically standing triangles at 410 cm−1 and 383 cm−1 corresponding to A1g and E12g respectively25,26,27. Comparing to the flat monolayer MoS2 on sapphire, the intensity ratio between A1g and E12g of the vertically standing triangles is higher, revealing a higher density of exposed edges in those vertically standing triangles. Figure 2d,e are the XPS spectra of Mo 3d and S 2p peak. The Mo 3d shows two peaks at 232.5 eV and 229.2 eV. The peaks, corresponding to the S 2p1/2 and S 2p3/2 orbital of divalent sulfide ions (S2−), are observed at 163.3 and 162 eV. These results agree well with the reported values for MoS2 crystal23,28,29.
Due to the anisotropic bonding and the general tendency to minimize the surface energy, nanoparticles of layer materials usually exhibit platelet-like morphology30,31. Alternatively, vertically standing triangles can also be obtained by a fast growth process32, the synthesis rate is mainly affected by the diffusion of product gas on the surface of the substrate. By controlling the reactant concentration, we can obtain the MoS2 films with different morphologies. Through regulating the carrier gas flow rate, the sulfidation rate of MoO3 can be controlled well. In addition, by changing the carrier gas flow rate, we can have a good understand of the growth process of MoS2 film.
As shown in Fig. 3a, when the carrier gas flow rate is 100sccm, the concentration of sulfidation vapor is too low to meet the needs. Meanwhile, with a slow carrier gas flow rate, the forming of MoO3−x is limited, and the transport rate of MoO3−x to the surface of substrate is also affected. In this condition, there are only some nanoparticles and some small rectangles on the surface of SiC, because of the lacking of S. So we increasing the carrier gas flow rate to 180sccm, the size of the rectangles increased, but we still do not obtained the vertically standing triangles. When the carrier gas flow increased to 260sccm, the synthesis rate is faster with the increasing of sulfidation concentration. Under this condition, we obtained the vertically standing triangles on the surface of SiC, the result is shown in Fig. 3c. Some of flat triangles were observed on the surface of SiC, only a few vertically standing triangles were obtained. So we increased the carrier gas flow to 340sccm, almost all of the triangles are perpendicular to the substrate, as shown in Fig. 3d. Figure 3e,f were the SEM images of flat rectangles and triangles. Figure 3f–h show the SEM images of vertically standing triangles. From the results, we can see that, only under a fast growth rate, can we get the vertically standing triangles of MoS2.
To have a better understand, the growth model is shown in Fig. 4. At the beginning of the growth, there was neither buffer layer nor seed on the surface of the substrate, so nucleation process was a 3D. The triangles are all coming from the islands of Fig. 4a. When the carrier gas flow rate is small, the atoms and molecules have enough time to mobility and diffusion on the surface of the substrate, so the synthesis process of MoS2 will be a 2D growth. With the supply of the sulfur vapor, the small islands grew into larger domain size, at last, the flat triangles of MoS2 were obtained, as shown in Fig. 4b. When the carrier gas flow rate is high enough, the chemical conversion occurs much faster than the diffusion of sulfur gas into the film. Under this condition, the sulfidation process will be rate-limiting. Meanwhile, with the anisotropic structure, the diffusion along the layers through van der Waals gaps is much faster than diffusion across the layers. Accordingly, the layers naturally orient perpendicular to the film, exposing van der Waals gaps for fast reaction. In this condition, the vertically structure formed, as shown in Fig. 4c.
As we know, the wettability has a great effect to the nucleation process at the begining of the growth. If the wettability of the subbstrate is good, the film will be a two dimmendional growth, along the surface of the subbstrate, then flat film will be obtained. If the wettability of the subbstrate is poor, the film will maintain a 3D growth along the layers. In order to prove our conclusion, and have a contrast, we also synthesis the MoS2 film on sapphire with the same condition: the temperature of MoO3 powder is 1000 °C, carrier gas flow rate were 100sccm, 180sccm, 260sccm, 340sccm respectively. The results were shown in Fig. 5. we can see that, the film on the sapphire are all flat triangles. There is no vertically standing triangles obtained on the sapphire. This is because the perfect wettability of MoS2 on the sapphire, the growth process will be 2D. Under this condition, there will not be vertically standing triangles formed. In contrast, with a poor wettability between MoS2 and SiC, the nucleation of the film on the surface of SiC is more difficult, and the growth process will be 3D, which is useful to synthesis the vertically standing triangles. Through the results, we can see that the wettability is also a important factor during the growth of the vertically standing MoS2.
HER catalytic activity of vertically standing MoS2 triangles on SiC was tested. Typical cathodic polarization curves and corresponding Tafel plots are shown in Fig. 6a,b. The Tafel slope in our vertically standing triangles was about 54 mV/decade. The related reports about vertically standing structures of MoS2 is about 94 mV/dec10 and 105–120 mV/dec23. Tafel plots are commonly used to evaluate the efficiency of the catalytic reaction. which indicated that the surface coverage of absorbed hydrogen was relatively low. The small Tafel plots means the high efficiency of the reaction. This indicating a good catalytic property of vertically standing triangles on SiC.
Conclusions
We have developed a rapid sulfidation process for the synthesis of vertically standing MoS2 triangles. SEM images reveal the synthesis mechanism of the triangles. It is suggested that under a high concentration of sulfur, the growth process will by a 3D growth, all these vertically standing triangles come from the small islands on the surface of SiC. In addition, by a comparision between the films grown on sapphire and SiC, we find the wettability is another factor for the forming of vertically standing triangles. At last, the HER properties of the triangles was tested. The Tafel slope is about 54 mV/decade, which is much smaller than the related reports about vertically standing MoS2 nanosheets.
Additional Information
How to cite this article: Lan, F. et al. Synthesis of Vertically Standing MoS2 Triangles on SiC. Sci. Rep. 6, 31980; doi: 10.1038/srep31980 (2016).
References
R. Tenne, L. Margulis, M. Genut & G. Hodes . Polyhedral & cylindrical structures of tungsten disulphide. Nature 360, 444–446 (1992).
Y. Feldman, E. Wasserman, D. J. Srolovitz & R. Tenne . High-Rate, Gas-Phase Growth of MoS2 Nested Inorganic Fullerenes & Nanotubes. Science 267, 222–225 (1995).
Y. Golan et al. Microtribology & Direct Force Measurement of WS2 Nested Fullerene-Like Nanostructures. Adv. Mater 11, 934–937 (1999).
J. Huang, S. Somu & A. Busnaina . A molybdenum disulfide/carbon nanotube heterogeneous complementary inverter. Nanotechnology 23, 5203–5208 (2012).
Y. Zhang, J. Ye, Y. Matsuhashi & Y. Iwasa . Ambipolar MoS2 thin flake transistors. Nano Lett 12, 1136–1140 (2012).
H. Liu, A. T. Neal & Peide D. Ye . Channel length scaling of MoS2 MOSFETs. ACS NANO 6, 8563–8569 (2012).
Edney, G. S. Firmiano et al. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst. Chem. Commun 48, 7687–7689 (2012).
B. Hinnemmann et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc 127, 5308–5309 (2005).
T. F. Jaramillo et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
D. Kong et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett 13, 1341–1347 (2013).
T. F. J. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
B. H. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
D. Kong et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
H. I. Karunadasa et al. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335, 698–702 (2012).
D. Merki, S. Fierro & X. Hu . Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 3, 2515–2525 (2012).
J. Wilcoxon, T. Thurston & J. Martin . Applications of metal and semiconductor nanoclusters as thermal and photo-catalysts. Nanostruct Mater 12, 993–997 (1999).
J. Lauritsen et al. Atomic-scale structure of Co–Mo–S nanoclusters in hydrotreating catalysts. J. Catal 197, 1–5 (2001).
T. Todorova, R. Prins & T. Weber . A density functional theory study of the hydrogenolysis and elimination reactions of C2H5SH on the catalytically active (100) edge of 2H-MoS2 . J. Catal 246, 109–117 (2007).
P. G. Moses, B. Hinnemann, H. Topsoe & J. K. Norskov . The hydrogenation and direct desulfurization reaction pathway in thiophene hydrodesulfurization over MoS2 catalysts at realistic conditions: a density functional study. J. Catal 248, 188–203 (2007).
J. V. Lauritsen et al. Location and coordination of promoter atoms in Co-and Ni-promoted MoS2-based hydrotreating catalysts. J. Catal 249, 220–233 (2007).
P. Raybaud et al. Ab initio study of the H2–H2S/MoS2 gas–solid interface: The nature of the catalytically active sites. J. Catal 189, 129–146 (2000).
J. Lauritsen et al. Atomic-scale insight into structure and morphology changes of MoS2 nanoclusters in hydrotreating catalysts. J. Catal 221, 510–522 (2004).
H. Li, H. Wu, Sh Yuan & He Qian . Synthesis and characterization of vertically standing MoS2 nanosheets. Sci. Rep. 6, 2117–2124 (2016).
M. Chen et al. TMD FinFET with 4 nm thin body and back gate control for future low power technology. IEEE. 978, 4673–9894 (2015).
Bret, C. Windom, W. G. Sawyer & David, W. Hahn . A Raman spectroscopic study of MoS2 and MoO3: applications to tribological systems. Tribol Lett. 42, 301–310 (2011).
H. Li et al. From bulk to monolayer MoS2: evolution of Raman scattering. Adv. Funct. Mater 22, 1385–1390 (2012).
B. Chakraborty, H. S. S. R. Matte, A. K. Sood & C. N. R. Rao . Layer-dependent resonant Raman scattering of a few layer MoS2 . Raman Spectrosc. 44, 92–96 (2013).
K. Lee et al. Electrical Characteristics of Molybdenum Disulfide Flakes Produced by Liquid Exfoliation. Adv. Mater. 23, 4178–4182 (2011).
Zh Lu et al. In situ fabrication of porous MoS2 thin-films as high-performance catalysts for electrochemical hydrogen evolution. Chemical Communication. 10, 1039–1042 (2013).
D. Kong et al. Few-layer nanoplates of Bi2Se3 and Bi2Te3 with highly tunable chemical potential. Nano Lett. 10, 2245–2250 (2010).
Y. Li et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. Am. Chem. Soc. 133, 7296–7299 (2011).
Y. Cheng et al. Van der Waals epitaxial growth of MoS2 on SiO2/Si by chemical vapor deposition. RSC Adv. 3, 17287–17293 (2013).
Author information
Authors and Affiliations
Contributions
Z.L., Y.X. and H.C. H.J. conceived the experiments. F.L., Z.W. and C.Q. synthesized the MoS2 flakes. J.C. and S.Z. perofomed the characterizations of Raman, SEM, optical microscope and HER catalytic activity. F.L. and Y.X. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lan, F., Lai, Z., Xu, Y. et al. Synthesis of Vertically Standing MoS2 Triangles on SiC. Sci Rep 6, 31980 (2016). https://doi.org/10.1038/srep31980
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31980
This article is cited by
-
Effect of Substrate symmetry on the dendrite morphology of MoS2 Film synthesized by CVD
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.