Abstract
The concentrations of methoxylated polychlorinated biphenyls (MeO-PCBs) and hydroxylated polychlorinated biphenyls (OH-PCBs) were measured in the sewage sludge samples collected from twelve wastewater treatment plants in China. Two MeO-PCB congeners, including 3′-MeO-CB-65 and 4′-MeO-CB-101, were detected in three sludge with mean concentrations of 0.58 and 0.52 ng/g dry weight, respectively. OH-PCBs were detected in eight sludge samples, with an average total concentration of 4.2 ng/g dry weight. Furthermore, laboratory exposure was conducted to determine the possible source of OH-PCBs and MeO-PCBs in the sewage sludge and their metabolism by the microbes. Both 4′-OH-CB-101 and 4′-MeO-CB-101 were detected as metabolites of CB-101 at a limited conversion rate after 5 days. Importantly, microbial interconversion between OH-PCBs and MeO-PCBs was observed in sewage sludge. Demethylation of MeO-PCBs was favored over methylation of OH-PCBs. The abundant and diverse microbes in sludge play a key role in the transformation processes of the PCB analogues. To our knowledge, this is the first report on MeO-PCBs in environmental matrices and on OH-PCBs in sewage sludge. The findings are important to understand the environmental fate of PCBs.
Similar content being viewed by others
Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous and persistent organic pollutants that are toxic to biota and humans1. Once released into the environment, PCBs are susceptible to a variety of transformation pathways2,3. With increasing scientific interest, the hydroxylated metabolites of PCBs (OH-PCBs) have been considered as environmental contaminants and detected in various media, including biotic samples (peregrine falcons, snapping turtles, polar bears and bowhead whales)4,5,6,7 and abiotic samples (air, surface water, precipitation and sediment)8,9,10. Furthermore, OH-PCBs in human blood reached a level comparable to PCBs11,12. In vivo and in vitro studies using animal and plant models have confirmed the transformation from PCBs to OH-PCBs13,14,15,16. OH-PCBs are generally considered to form via oxidative mechanisms, such as epoxide intermediates and the direct insertion of the hydroxyl group into a biphenyl, which is mediated by cytochrome P450 enzymes17,18. For most toxicological endpoints, OH-PCBs are more toxic than their parent PCBs, which affect the endocrine system, brain development and reproductive processes19,20.
Recently, we reported the formation of methoxylated metabolites of PCBs (MeO-PCBs) in intact rice plants via laboratory exposure21. The interconversion between OH-PCBs and MeO-PCBs was also observed. These results suggested that the generation of MeO-PCBs and their interconversion with OH-PCBs are important metabolic pathways that nevertheless have been ignored. To date, there is no information regarding the occurrence of MeO-PCBs in the environment. Their chemical structures suggest that MeO-PCBs are possibly more lipophilic and persistent than OH-PCBs. Thus, it is important to determine the presence and behavior of newly identified MeO-PCBs other than OH-PCBs in the environment.
Previous studies have reported that a variety of organic pollutants, such as PCBs and polybrominated diphenyl ethers (PBDE)22,23, have been detected at relatively high concentrations in sewage sludge from wastewater treatment plants (WWTPs), indicating WWTPs are important sinks and secondary emission sources of these chemicals to the ambient environment24,25. There are currently no published data on the levels of OH-PCBs and MeO-PCBs in sewage sludge. A previous study supposed that OH-PCBs in surface waters that were collected near WWTPs might be the result of microbial oxidation or other oxidation treatment processes9, which however, has not been verified. Natural microbes play an important role in the biotransformation of various pollutants26,27. The hydroxylation of PCBs is a step in the PCB degradation process of white-rot fungi28. Various bacterial groups are capable of degrading PCBs via aerobic oxidative processes and anaerobic reductive processes29,30. As a complex matrix, sewage sludge contains a large number of microorganisms that can cause diverse transformation of PCBs31. Sewage sludge may be used to track the presence and fate of MeO-PCBs and OH-PCBs on a large geographical scale.
In the present work, sewage sludge samples were collected from 12 cities in China to provide valuable information on the presence, distribution and potential sources of MeO-PCBs and OH-PCBs in the environment. We further investigated the microbial transformation of PCB, MeO-PCB and OH-PCB congeners in a laboratory-simulated environment. This study reported the presence of MeO-PCBs in the environment and proposed possible microbial transformation between MeO-PCBs and OH-PCBs in sewage sludge.
Results and Discussion
Concentrations and compositions of MeO-PCBs and OH-PCBs in sludge
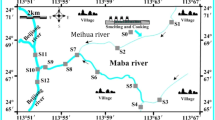
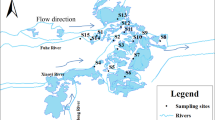
The measured concentrations of the targeted analytes in the sewage sludge are summarized in Table 1. The spatial distributions of ΣPCBs, ΣOH-PCBs and ΣMeO-PCBs in the sewage sludge from different sampling locations in Greater China are shown in Fig. 1. In this study, two MeO-PCB congeners, 3′-MeO-CB-65 and 4′-MeO-CB-101, were detected in the sewage sludge samples from WWTPs in Zhejiang Province, Guangdong Province and Shanghai Municipality. The concentrations ranges of 3′-MeO-CB-65 and 4′-MeO-CB-101 were 0.41–0.89 ng/g and 0.43–0.65 ng/g, respectively.
Sampling locations and spatial distributions of the concentrations of PCBs, OH-PCBs and MeO-PCBs in the sewage sludge samples from twelve wastewater treatment plants in Greater China.
The map was produced on ArcGIS 10.3.1 (http://www.esri.com/).
OH-PCBs were found in 8 sludge samples with a detection rate of 67%. Four OH-PCB congeners, including 3′-OH-CB-65, 4′-OH-CB-101, 4′-OH-CB-18 and 4′-OH-CB-26, were identified. The total concentrations of the OH-PCBs ranged from <0.1 to 11.5 ng/g, with a mean of 4.23 ng/g. The dominant congeners were 3′-OH-CB-65 (mean 49% of the total concentration of OH-PCBs) and 4′-OH-CB-101 (mean 33% of the total concentration of OH-PCBs). The number of reports on the occurrence of OH-PCBs in abiotic samples is limited. The detected concentrations of OH-PCBs in the sludge of the present study were comparable to those detected in the sediment from Lake Michigan, USA (0.20 to 26 ng/g with a mean of 8.5 ng/g)10.
To investigate the relationships among PCBs, OH-PCBs and MeO-PCBs, the contamination status of the PCBs in the sludge samples was determined. The total concentrations of the PCBs in the sludge samples ranged from 3.0 to 170 ng/g with a mean of 35.8 ng/g and a detection rate of 100%. The dominant PCBs were CB-28, 52 and 101, which were commonly used to indicate the PCB contamination in the environment32,33. The results show that low-chlorinated PCBs are the major PCB homologue group residing in sewage sludge in Chinese WWTPs. There were also several peaks of unknown compounds detected in the chromatograms, which might be the metabolites of CB-28, CB-52 or other PCBs, though none of them were identified due to the lack of authentic standards at the time of analysis. This study focused on only ten low-chlorinated OH-PCBs that were found in sediment and commercial PCB mixtures, together with ten homologous MeO-PCBs. More research is needed to identify more potential OH-PCBs and MeO-PCBs in the ambient environment.
The PCBs, OH-PCBs and MeO-PCBs showed higher levels in Zhejiang Province, Shanghai Municipality and Guangdong Province. The concentration of PCBs in the surface soil of Shanghai Municipality was found higher than other regions of China34. In Zhejiang and Guangdong Provinces, electronic waste recycling activities are considered a very important emission source of PCBs35,36. The concentrations of the ΣPCBs were significantly correlated with those of the ΣOH-PCBs (R = 0.755, p < 0.01) and ΣMeO-PCBs (R = 0.762, p < 0.01). The concentrations of the ΣOH-PCBs were also significantly correlated with those of the ΣMeO-PCBs (R = 0.776, p < 0.01). For the individual homologous congeners, the concentrations of 3′-OH-CB-65 were significantly correlated with those of 3′-MeO-CB-65 (R = 0.791, p < 0.05). Close correlations were found among 4′-OH-CB-101, 4′-MeO-CB-101 and CB-101 (R = 0.762–0.839, p < 0.01). The concentrations of high chlorinated CB-153 were also significantly correlated with those of low chlorinated 4′-OH-PCB-101 and 4′-MeO-PCB-101 (R = 0.895–0.946, p < 0.01). A strong correlation between concentrations of two contaminants may suggest transformation relationships and/or common sources. No significant relationship was observed between the concentrations of OH-PCB and the total organic carbon (TOC) content, or between the concentrations of MeO-PCBs and the TOC content in the sludge samples (p > 0.05).
The highest levels of the three compound groups were all in the WWTP from Zhejiang Province. This WWTP, which was located near an electronic waste dismantling area, treated a mixture of domestic and industrial wastewater. Influent and effluent samples in this WWTP were analyzed to explore the possible source of the analytes (i.e., OH-PCBs and MeO-PCBs). PCBs were detected in the suspended particulate matter (SPM) of the influent and effluent at concentrations of 6.9 ng/g and 1.3 ng/g, respectively. PCBs were also found in water of the influent and effluent with concentrations of 679 pg/L and 141 pg/L, respectively. The concentrations of PCBs in the sludge were higher than those in the wastewater. Only one OH-PCB congener, i.e., 3′-OH-CB-65, was identified in the influent, with concentrations of 0.9 ng/g in the SPM and 65 pg/L in the water. MeO-PCBs were not found in the influent and effluent samples, corroborating their formation in sludge.
The hypothetical precursor of 3′-OH-CB-65 and 3′-MeO-CB-65, namely CB-65, was not found in the sludge or wastewater, suggesting that 3′-OH-CB-65 and 3′-MeO-CB-65 might not be formed as the metabolite of CB-65 in the wastewater treatment process. A previous study reported the presence of several OH-PCBs in the original Aroclors and found 3′-OH-PCB-65 to be the most prominent congener in Aroclors 1221, 1242, 1248 and 125410. This indicated that the accumulation of 3′-OH-CB-65 in sewage sludge was at least partially due to OH-PCB contamination of the original Aroclors. Although PCBs have been banned for use, there was still considerable emission from the disposal of PCB-containing materials37. Therefore, WWTPs were possible receivers of PCBs and the coexisting OH-PCBs. The persistence of 3′-OH-CB-65 should also be concerned since it was recently detected in the sediment of the Lake Michigan. 4′-OH-CB-101, 4′-MeO-CB-101 and their parent compound, CB-101, were all detected in the sludge samples. In our previous study where rice plants were used as the model, CB-61 was biotransformed to 4′-OH-CB-61 (major metabolite) and 4′-MeO-CB-61 (minor metabolite)21. Moreover, the interconversion between OH-PCBs and MeO-PCBs is an important metabolic pathway. On the basis of these observations, we hypothesized that microbes in sludge play a key role in the formation of OH-PCBs and MeO-PCBs. The results of the exposure study provide compelling evidence for this hypothesis.
Metabolism of PCBs, MeO-PCBs and OH-PCBs by microbes in sludge
The hydroxylated and methoxylated metabolites of CB-101 and CB-65 in exposed sludge were analyzed. The 4′-OH-CB-101 and 4′-MeO-CB-101 were identified after the sludge was exposed to CB-101, whereas 3′-OH-PCB-65 and 3′-MeO-PCB-65 were not detected as metabolites of CB-65 (Fig. 2). The metabolic properties of PCBs may depend on the number and location of chlorine atoms on the ring structure of the PCB molecule38. Moreover, the hydroxyl and methoxyl were likely to preferentially occur at the para position, which needs the least energy during enzymatic reaction39.
The interconversion between OH-PCBs and the related MeO-PCBs mediated by microbes in sludge was observed. Transformations from OH-PCBs to MeO-PCBs and from MeO-PCBs to OH-PCBs both occurred after sludge exposure (Fig. 2). The results were consistent with those of the plant exposure study, further supporting that the recently found metabolic pathway of PCBs is ubiquitous and may reflect real PCB biotransformation in the environment21. Moreover, similar hydroxylation and methoxylation pathways of PBDEs in plants and animals have been proposed in previous studies40,41,42. The comprehensive results show that a reciprocal transformation between hydroxylated and methoxylated metabolites of other compounds may also exist in the environment.
The conversion percentages, the mass of the metabolite after 5-day exposure over that of the initial parent compound (M/P), are shown in Table 2. The average M/P values were 1.65% for 4′-OH-CB-101/CB-101 and, 0.40% for 4′-MeO-CB-101/CB-101. The transformation rate of CB-101 in sludge was greater than that of CB-61 in rice plant21. The average M/P values were 1.51% for 3′-MeO-PCB-65/3′-OH-PCB-65 and 6.83% for 3′-OH-PCB-65/3′-MeO-PCB-65. The average M/P values were 1.75% for 4′-MeO-CB-101/4′-OH-CB-101 and 9.47% for 4′-OH-CB-101/4′-MeO-CB-101. Overall, the demethylation of MeO-PCBs was favored over the methylation of OH-PCBs. Consequently, the concentrations and detection rates of OH-PCBs were higher than those of MeO-PCBs in the collected sludge samples. This may also explain why OH-PCBs have been widely detected, whereas MeO-PCBs have never been observed in the environment. There might be co-elution of isomers in the analysis of sludge samples from WWTPs, which could not be completely avoided due to the large number of isomers and lack of authentic standards. However, the results of these exposure experiments, from some point, verified the detection of targeted compounds.
All the transformation processes occurred rapidly in the sludge and the metabolites were detected after exposure for only one day (Fig. 2). Generally, the mean residual amount of exposure compounds in sludge slightly decreased and the mean amount of metabolites gradually increased over 5 days, though no statistically significant difference between the different time points was observed (p > 0.05). The metabolism between OH-PCBs and MeO-PCBs may occur simultaneously in the sludge. The mean recoveries of these compounds ranged from 74% to 83% for the exposure groups. This indicated that these compounds may be utilized by microbes in sludge as carbon sources. Some other unknown metabolites, such as diOH-PCBs may also be generated, though none of them have been identified30.
None of the metabolites were found in the blank control, water control or sterile control, suggesting that the microbes in the sludge were responsible for the transformation of PCBs, OH-PCBs and MeO-PCBs. No cross-contamination was found between the reactors. The purity of the six exposure chemical standards was verified and there was no undesirable OH-PCB and MeO-PCB detected as impurities that would affect the metabolic results in this study. The metabolic results of PCBs, OH-PCBs and MeO-PCBs by microbes in sludge are illustrated in Fig. 3. MeO-PCBs may be reaction intermediates in the formation of OH-PCBs from PCBs, making them difficult to detect in the environment. MeO-PCBs may also be the final transformation product of OH-PCBs, though the conversion rate was relatively low.
Source estimation and environmental implications
Two major reasons for that MeO-PCBs and OH-PCBs were found in the collected sludge samples in this study are as follows: (i) the levels of PCBs were relatively high in some selected WWTPs, such as in Zhejiang Province, Shanghai Municipality and Guangdong Province; and (ii) abundant and diverse microbes existed in the sewage sludge, which is a special medium and functioned in the entire metabolic process of PCBs, OH-PCBs and MeO-PCBs. Several Studies show that anaerobic and aerobic processes mediated PCBs degradation. Highly chlorinated PCBs were removed chlorine atoms under anaerobic process and then mineralized under the aerobic condition. The factors influencing the transformation included the complexity of the PCB congener, the type of microorganism employed and the interaction among the microorganisms29,30. Our ongoing studies include exploring the key microbe species that are involved in the proposed metabolic pathway in this work.
4′-OH-CB-101 has been found as a major metabolite in various animals43,44. Although the concentration of 4′-OH-CB-101 was below the detection limit in the influent sample, we could not exclude the possibility that a proportion of 4′-OH-CB-101 might be formed by humans at trace concentration and entered the WWTPs from human excretion. The 4′-MeO-CB-101 could be generated from both CB-101 and 4′-OH-CB-101 by microbes in the sludge. The 4′-MeO-CB-101 was also a potential intermediate that requires further confirmation.
Compared with the conversion rate in the present exposure study, the calculated rates of concentration of both MeO-PCB/PCB and MeO-PCB/OH-PCB were higher in the sludge samples collected from the WWTPs. There are several reasons can explain this. First, the microbial reaction in the WWTPs might be more active than in the laboratory. Second, a portion of the parent compounds in the sewage might be discharged with the effluent without full contact with the sludge. Finally, hydrophobic MeO-PCBs were more easily preserved in the sludge than OH-PCBs. This work was not meant to establish the WWTP sludge as the only source of MeO-PCBs and OH-PCBs; however, it is one source. Previous studies have shown that sludge amendment can be a source of elevated levels of a variety of pollutants to agricultural soils45. The presence of OH-PCBs and MeO-PCBs in the sewage sludge may therefore be another cause for concern.
In summary, this is the first study on the detection of MeO-PCBs and OH-PCBs in sewage sludge, which is important because MeO-PCBs are a class of previously undiscovered chemicals in the environment. Microbes in sewage sludge play a key role in the transformation of the PCB analogues, including the hydroxylation and methoxylation of PCBs, as well as the interconversion between OH-PCBs and MeO-PCBs. Wastewater treatment plants are overlooked producers of widespread OH-PCBs in the environment. Other than microbial transformation, the potential sources of OH-PCBs and MeO-PCBs in sewage sludge also include the accumulation from original commercial Aroclors and human excretion. Wastewater treatment plants are a possible emission source of OH-PCBs and MeO-PCBs to the surrounding environment.
Experimental section
Materials
The low-chlorinated PCBs (mainly di- to penta-PCBs) are the major PCB homologue group residing in the environment in China34. Accordingly, the selected OH-PCB analytes in this study were mainly low-chlorinated congeners, which have been found in sediment samples and original commercial Aroclors at relatively high concentrations10. The homologous MeO-PCBs were also selected as targeted analytes. The full names, abbreviations and chemical structures for the target compounds are shown in Table S1. Although there are 837 pairs of theoretically possible OH-PCBs and MeO-PCBs, the commercially available homologous OH-PCBs and MeO-PCBs are limited at the time of analysis. The ten standards of OH-PCBs were 2′-OH-CB-12, 4-OH-CB-14, 4′-OH-CB-18, 4′-OH-CB-26, 2′-OH-CB-61, 3′-OH-CB-61, 4′-OH-CB-61, 2′-OH-CB-65, 3′-OH-CB-65 and 4′-OH-CB-101. The ten standards of MeO-PCBs were 2′-MeO-CB-12, 4-MeO-CB-14, 4′-MeO-CB-18, 4′-MeO-CB-26, 2′-MeO-CB-61, 3′-MeO-CB-61, 4′-MeO-CB-61, 2′-MeO-CB-65, 3′-MeO-CB-65 and 4′-MeO-CB-101. The ten studied PCBs were CB-18, 26, 28, 52, 61, 65, 101, 138, 153 and 180. The surrogate standards were 4′-MeO-CB-159 and 4′-OH-CB-159 for the neutral and phenolic chemicals, respectively10. Acetonitrile, methyl tert-butyl ether (MTBE), hexane, acetone and dichloromethane (DCM) were of HPLC grade or pesticide grade. Silica gel and anhydrous sodium sulfate were activated in advance. Acidified silica gel was prepared by mixing activated silica (70 g) with concentrated H2SO4 (30 g). Deionized water (18.2 MΩ) was generated via a Milli-Q system.
Sample collection
The sampling map and sites are shown in Fig. 1. A total of 12 sewage sludge samples were collected from September 2013 to June 2014 from different WWTPs within 12 provinces and municipalities in Greater China. Detailed information on the WWTP characteristics is provided in Table S2. The freshly digested sludge samples (approximately 1 kg for each sample) from the dewatering process were packed in aluminum foil, sealed in kraft bags and immediately delivered to a laboratory. The samples were then freeze-dried, homogenized, sieved through a stainless steel 100-mesh sieve and preserved at −20 °C until analysis. Two grams of each sludge sample were used in the determination experiments. The calculations of the concentrations of the targeted compounds in the sludge were based on the dry weight (dw). The influent and effluent water samples (approximately 1 L for each sample) were taken from a WWTP in Zhejiang Province in September 2014, from which a sludge sample was previously collected. These water samples were extracted promptly after centrifugation at 5000 rpm for 10 min and the entire remaining SPM was also collected for further analysis.
Laboratory-simulated sludge exposure
Laboratory-based exposure studies were conducted to identify the possible reason for the occurrence of the targeted chemicals in the sewage sludge. Based on the results of the field investigation, two PCB congeners, CB-65 and CB-101, two MeO-PCB congeners, 3′-MeO-CB-65 and 4′-MeO-CB-101 and two OH-PCB congeners, 3′-OH-CB-65 and 4′-OH-CB-101, were selected as the exposure compounds. The six compounds were added separately (10 μg) to 65 mL of laboratory-simulated sewage sludge and mixed thoroughly in a 100 mL brown incubator bottle. Seed sludge (15 mL) was added to each of the incubator bottles as a cosubstrate to begin the digestion46. The sewage consisted of yeast extract, meat extract, peptone, urea, (NH4)2SO4, K2HPO4, CaCl2, MgSO4 and trace element solution47.
The blank control (in the absence of the exposure compounds), water control (exposure compounds only in deionized water) and the sterile control (exposure compounds in fully sterile sludge) were prepared similarly to the exposure groups. Each of the bottles was placed simultaneously on an incubated shaker-table at 35 ± 2 °C that was kept under the same conditions. The total exposure time was 5 days, which was similar to the common sludge retention time in WWTPs. The sludge of the exposure group was sampled at intervals of 1, 2, 3, 4 and 5 days. At the end of the exposure time, the control groups were sampled. The exposure and control groups were prepared in triplicate, including the ones for different time intervals. The sludge was freeze-dried, homogenized and stored at −20 °C prior to analysis. No targeted compounds existed in the simulated sludge that was used in this study before the exposure experiment.
Sample pretreatment and analysis
The sample pretreatment and analysis were adapted from the previously reported method21. Briefly, the solid sample (sludge and SPM) was spiked with surrogate standards and ultrasonically extracted for 60 min twice using hexane/MTBE (1:1 v/v; 40 mL). The extracts were combined and evaporated to dryness and redissolved in 50 mL of DCM. Acidified silica gel (10 g) was added and the mixture was shaken vigorously for 10 min to remove the lipids. The acidified silica gel was then removed via an anhydrous Na2SO4 column (15 g). An additional 40 mL of DCM was used to further elute the compounds. A secondary purification cycle was performed following the same operational steps. Then, sulfur was eliminated by the addition of activated copper powder (2 g). The extract was concentrated to dryness and dissolved with 400 μL hexane. A half of the extract was transferred into a vial for subsequent analysis of the PCBs and MeO-PCBs by gas chromatography/mass spectrometry (GC/MS). The other half of the extract was dried under a nitrogen stream and redissolved in 200 μL of acetonitrile for analyzing OH-PCBs with liquid chromatography/tandem mass spectrometry (LC/MS/MS). Without the commonly used prior derivatization of OH-PCBs17, the entire sample preparation was simplified with satisfying sensitivity of the method.
The water samples were extracted using a liquid–liquid extraction method. The water sample (1L) was spiked with surrogates, mixed with 100 mL of DCM and shaken for 10 min. Then, the DCM was transferred to another glass bottle and the extraction was repeated twice. The combined extract was concentrated by rotary evaporation to 50 mL and further purified and analyzed as described for the solid samples.
The quantitative analysis of the PCBs and MeO-PCBs was conducted on a 7890B/5977A GC/MS instrument (Agilent Technologies, Santa Clara, CA, USA) operated with electron impact source. The GC was fitted with a DB-5 MS capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA) with helium as the carrier gas at a constant flow rate of 1.0 mL/min. The oven temperature was initially set at 80 °C, ramped to 140 °C at 10 °C/min and increased to 300 °C at 2.5 °C/min. The selected ion monitoring (SIM) mode was used for the quantitative determination. The ions that were used to analyze the targeted MeO-PCBs and PCBs are listed in Table S3. The GC/MS chromatograms of PCB and MeO-PCB standards were presented in Figures S1 and S2. A GC with tandem MS (GC/MS/MS) (Agilent 7890B–7000C) was employed to further confirm the identification accuracy of MeO-PCBs. The precursor and product ions of the targeted MeO-PCBs are listed in Table S4.
The quantification of the OH-PCBs was performed on an Agilent 1260–6460 LC/MS/MS instrument. A C18 column (100 mm × 2.1 mm, 2.2 μm particle size, Thermo Fisher Scientific, Waltham, MA, USA) was chosen for chromatographic separation and quantification. The mobile phase consisted of acetonitrile and water, which was used with a gradient elution of a ratio ranging from 45:55 to 90:10 over 35 min at a flow rate of 0.3 mL/min. The MS was operated with a negative electrospray ionization (ESI) source in multiple-reaction monitoring (MRM) mode. Detailed information on the ion transitions that were monitored for each OH-PCB is provided in Table S5. The LC/MS/MS chromatograms of OH-PCB standards were presented in Figure S3. Another Agilent ZORBAX SB-C18 column (150 mm × 2.1 mm, 3.5 μm particle size) was used for further identification of the analytes. The corresponding mobile phase consisted of mehanol and water with a gradient elution of a ratio ranging from 55:45 to 85:15 over 50 min at a flow rate of 0.3 mL/min.
Quality assurance and quality control
All of the reported data were subject to strict quality assurance and control procedures. No mutual interference was observed in the instrumental analysis of the phenolic and neutral compounds. A procedural blank, a spiked blank and a sample duplicate were processed in parallel with each batch of six samples. The procedural blank, Na2SO4, was used to monitor for background contamination levels and all analytes were under the detection limits. The average recoveries of the PCBs, MeO-PCBs and OH-PCBs in the spiked samples were 81.3–90.9%, 80.5–91.4% and 73.2–104.2%, respectively, where the relative standard deviation (RSD) was lower than 18% (n = 3). The recoveries of the surrogate standards were 85.1–96.4% for 4′-MeO-CB-159 and 83.5–102.2% for 4′-OH-CB-159. Duplicates were included in the sludge sample analysis and the RSD of the detected concentration was lower than 15% (n = 3). The instrumental calibration was verified by injecting five calibration standards and the linearity of the calibration curve (R2) was > 0.99. The method limits of detection (MLODs) were calculated at a signal-to-noise ratio of 3. The MLODs for the PCBs, MeO-PCBs and OH-PCBs in the solid samples were 0.2–1.5, 0.1–1.2 and 0.05–0.8 ng/g, respectively. The MLODs for the three groups of compounds in water were 45–180, 60–200 and 20–80 pg/L, respectively. The statistical analysis including the Pearson’s correlation analysis was performed using SPSS 18.0 and Origin 8.0. Statistical significance was considered as p < 0.05.
Additional Information
How to cite this article: Sun, J. et al. Detection of methoxylated and hydroxylated polychlorinated biphenyls in sewage sludge in China with evidence for their microbial transformation. Sci. Rep. 6, 29782; doi: 10.1038/srep29782 (2016).
References
Nomiyama, K. et al. Toxicological assessment of polychlorinated biphenyls and their metabolites in the liver of Baikal seal (Pusa sibirica). Environ. Sci. Technol. 48, 13530–13539 (2014).
Grimm, F. A. et al. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 45, 245–272 (2015).
Letcher, R. J., Klasson-Wehler, E. & Bergman, A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. Handbook of Environmental Chemistry: New Types of Persistent Halogenated Compound 3K, 315–359 (2000).
Fernie, K. J. & Letcher, R. J. Historical contaminants, flame retardants and halogenated phenolic compounds in peregrine falcon (Falco peregrinus) nestlings in the Canadian Great Lakes Basin. Environ. Sci. Technol. 44, 3520–3526 (2010).
Letcher, R. J., Lu, Z., de Solla, S. R., Sandau, C. D. & Fernie, K. J. Snapping turtles (Chelydra serpentina) from Canadian Areas of Concern across the southern Laurentian Great Lakes: Chlorinated and brominated hydrocarbon contaminants and metabolites in relation to circulating concentrations of thyroxine and vitamin A. Environ. Res. 143, 266–278 (2015).
Gustayson, L. et al. Hydroxylated polychlorinated biphenyls decrease circulating steroids in female polar bears (Ursus maritimus). Environ. Res. 138, 191–201 (2015).
Hoekstra, P. F. et al. Hydroxylated and methylsulfone-containing metabolites of polychlorinated biphenyls in the plasma and blubber of bowhead whales (Balaena mysticetus). Environ. Toxicol. Chem. 22, 2650–2658 (2003).
Awad, A. M., Martinez, A., Marek, R. F. & Hornbuckle, K. C. Occurrence and distribution of two hydroxylated polychlorinated biphenyl congeners in Chicago air. Environ. Sci. Technol. Lett. 3, 47–51 (2016).
Ueno, D. et al. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ. Sci. Technol. 41, 1841–1848 (2007).
Marek, R. F., Martinez, A. & Hornbuckle, K. C. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a Lake Michigan waterway and original commercial Aroclors. Environ. Sci. Technol. 47, 8204–8210 (2013).
Eguchi, A. et al. Different profiles of anthropogenic and naturally produced organohalogen compounds in serum from residents living near a coastal area and e-waste recycling workers in India. Environ. Int. 47, 8–16 (2012).
Weiss, J. et al. Hydroxy-PCBs, PBDEs and HBCDDs in serum from an elderly population of Swedish fishermen’s wives and associations with bone density. Environ. Sci. Technol. 40, 6282–6289 (2006).
Routti, H. et al. Biotransformation of PCBs in relation to phase I and II xenobiotic-metabolizing enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea. Environ. Sci. Technol. 42, 8952–8958 (2008).
Sandala, G. M. et al. Hydroxylated and methyl sulfone PCB metabolites in adipose and whole blood of polar bear (Ursus maritimus) from east Greenland. Sci. Total Environ. 331, 125–141 (2004).
Lu, Z., Kania-Korwel, I., Lehmler, H. J. & Wong, C. S. Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 47, 12184–12192 (2013).
Zhai, G. S., Gutowski, S. M., Lehmler, H. J. & Schnoor, J. L. Enantioselective transport and biotransformation of chiral hydroxylated metabolites of polychlorinated biphenyls in whole poplar plants. Environ. Sci. Technol. 48, 12213–12220 (2014).
Liu, J. Y., Hu, D. F., Jiang, G. B. & Schnoor, J. L. In vivo biotransformation of 3,3′,4,4′-tetrachlorobiphenyl by whole plants-poplars and switchgrass. Environ. Sci. Technol. 43, 7503–7509 (2009).
Wu, X. N., Duffel, M. & Lehmler, H. J. Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem. Res. Toxicol. 26, 1642–1651 (2013).
Londono, M., Shimokawa, N., Miyazaki, W., Iwasaki, T. & Koibuchi, N. Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells. J. Appl. Toxicol. 30, 334–342 (2010).
Gabrielsen, K. M. et al. Levels and patterns of hydroxylated polychlorinated biphenyls (OH-PCBs) and their associations with thyroid hormones in hooded seal (Cystophora cristata) mother-pup pairs. Aquat. Toxicol. 105, 482–491 (2011).
Sun, J. T., Pan, L. L., Su, Z. Z., Zhan, Y. & Zhu, L. Z. Interconversion between methoxylated and hydroxylated polychlorinated biphenyls in rice plants: an important but overlooked metabolic pathway. Environ. Sci. Technol. 50, 3668–3675 (2016).
Wang, Y. W. et al. Effect of municipal sewage treatment plant effluent on bioaccumulation of polychlorinated biphenyls and polybrominated diphenyl ethers in the recipient water. Environ. Sci. Technol. 41, 6026–6032 (2007).
Sun, J. T. et al. Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) in biosolids from municipal wastewater treatment plants in China. Chemosphere 90, 2388–2395 (2013).
Gbondo-Tugbawa, S. S., McAlear, J. A., Driscoll, C. T. & Sharpe, C. W. Total and methyl mercury transformations and mass loadings within a wastewater treatment plant and the impact of the effluent discharge to an alkaline hypereutrophic lake. Water Res. 44, 2863–2875 (2010).
Rubirola, A. et al. Characterization of metoprolol biodegradation and its transformation products generated in activated sludge batch experiments and in full scale WWTPs. Water Res. 63, 21–32 (2014).
Cooper, M. et al. Anaerobic microbial transformation of halogenated aromatics and fate prediction using electron density modeling. Environ. Sci. Technol. 49, 6018–6028 (2015).
Homme, C. L. & Sharp, J. O. Differential microbial transformation of nitrosamines by an inducible propane monooxygenase. Environ. Sci. Technol. 47, 7388–7395 (2013).
Kamei, I., Kogura, R. & Kondo, R. Metabolism of 4,4′-dichlorobiphenyl by white-rot fungi Phanerochaete chrysosporium and Phanerochaete sp MZ142. Appl. Microbiol. Biotechnol. 72, 566–575 (2006).
Mackova, M. et al. Bacterial degradation of polychlorinated biphenyls. Geomicrobiology: Molecular and Environmental Perspective 347–366 (2010).
Borja, J., Taleon, D. M., Auresenia, J. & Gallardo, S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 40, 1999–2013 (2005).
Jiang, X. B. et al. Efficient nitro reduction and dechlorination of 2,4-dinitrochlorobenzene through the integration of bioelectrochemical system into upflow anaerobic sludge blanket: A comprehensive study. Water Res. 88, 257–265 (2016).
Pavlova, P. A. et al. Polychlorinated biphenyls in a temperate alpine glacier: 1. Effect of percolating meltwater on their distribution in glacier ice. Environ. Sci. Technol. 49, 14085–14091 (2015).
Tu, C. et al. Potential for biodegradation of polychlorinated biphenyls (PCBs) by Sinorhizobium meliloti. J. Hazard. Mater. 186, 1438–1444 (2011).
Ren, N. Q. et al. Polychlorinated biphenyls in chinese surface soils. Environ. Sci. Technol. 41, 3871–3876 (2007).
Wang, P. et al. Temporal trends of PCBs, PCDD/Fs and PBDEs in soils from an E-waste dismantling area in East China. Environ. Sci.: Proc Impacts 15, 1897–1903 (2013).
Zheng, J. et al. Polychlorinated biphenyls (PCBs) in human hair and serum from e-waste recycling workers in southern China: Concentrations, chiral signatures, correlations and source identification. Environ. Sci. Technol. 50, 1579–1586 (2016).
Herrick, R. F., Lefkowitz, D. J. & Weymouth, G. A. Soil contamination from PCB-containing buildings. Environ. Health Perspect. 115, 173–175 (2007).
Rezek, J., Macek, T., Mackova, M. & Triska, J. Plant metabolites of polychlorinated biphenyls in hairy root culture of black nightshade Solanum nigrum SNC-90. Chemosphere 69, 1221–1227 (2007).
Ma, C. X. et al. Identification of a novel hydroxylated metabolite of 2,2′,3,5′,6-pentachlorobiphenyl formed in whole poplar plants. Environ. Sci. Pollut. Res. 23, 2089–2098 (2016).
Sun, J. T. et al. In vivo metabolism of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in young whole pumpkin plant. Environ. Sci. Technol. 47, 3701–3707 (2013).
Sun, J. T., Liu, J. Y., Liu, Y. W., Yu, M. & Jiang, G. B. Reciprocal transformation between hydroxylated and methoxylated polybrominated diphenyl ethers in young whole pumpkin plants. Environ. Sci. Technol. Lett. 1, 236–241 (2014).
Wan, Y. et al. Interconversion of hydroxylated and methoxylated polybrominated diphenyl ethers in Japanese medaka. Environ. Sci. Technol. 44, 8729–8735 (2010).
Imaeda, D. et al. Blood levels of polychlorinated biphenyls and their hydroxylated metabolites in Baikal seals (Pusa sibirica): Emphasis on interspecies comparison, gender difference and association with blood thyroid hormone levels. Chemosphere 114, 1–8 (2014).
Kunisue, T., Sakiyama, T., Yamada, T. K., Takahashi, S. & Tanabe, S. Occurrence of hydroxylated polychlorinated biphenyls in the brain of cetaceans stranded along the Japanese coast. Mar. Pollut. Bull. 54, 963–973 (2007).
Ejarrat, E., Marsh, G., Labandeira, A. & Barcelo, D. Effect of sewage sludges contaminated with polybrominated diphenylethers on agricultural soils. Chemosphere 71, 1079–1086 (2008).
Sverko, E. et al. Evidence for anaerobic dechlorination of dechlorane plus in sewage sludge. Environ. Sci. Technol. 49, 13862–13867 (2015).
Bellucci, M., Ofiteru, I. D., Head, I. M., Curtis, T. P. & Graham, D. W. Nitrification in hybrid bioreactors treating simulated domestic wastewater. J. Appl. Microbiol. 115, 621–630 (2013).
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (21520102009, 21137003 and 21507111), National Basic Research Program of China (973 Program, 2014CB441101), Zhejiang Provincial Natural Science Foundation of China (LY14B070009) and the Fundamental Research Funds for the Central Universities (2016FZA6007). We thank Xiaodan Wu from the Analysis and Measurement Center of Zhejiang University for assistance in sample analysis.
Author information
Authors and Affiliations
Contributions
J.S. and L.Z. designed research and wrote the manuscript. Z.W., Y.S., Y.Z. and L.Q. conducted the experiments. J.S., L.P. and Y.Z. analyzed data. All authors have read and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, J., Zhu, L., Pan, L. et al. Detection of methoxylated and hydroxylated polychlorinated biphenyls in sewage sludge in China with evidence for their microbial transformation. Sci Rep 6, 29782 (2016). https://doi.org/10.1038/srep29782
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29782
This article is cited by
-
Photolytic transformations of polychlorobiphenyls
Russian Chemical Bulletin (2023)
-
PCB-degradation kinetics of three fungal isolates and their consortium from paint scrape-contaminated site
Environmental Sustainability (2022)
-
A semi-target analytical method for quantification of OH-PCBs in environmental samples
Environmental Science and Pollution Research (2020)
-
Immobilizing Laccase on Different Species Wood Biochar to Remove the Chlorinated Biphenyl in Wastewater
Scientific Reports (2018)
-
Uptake, translocation, and metabolism of hydroxylated and methoxylated polychlorinated biphenyls in maize, wheat, and rice
Environmental Science and Pollution Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.