Abstract
Lipopolysaccharides (LPS), integral components of the outer membrane of all gram-negative bacteria, are closely associated with foodborne diseases such as fever, diarrhea and hypotension and thus, the early and sensitive detection of LPS is necessary. In this study, an aptasensor assay based on hybridization chain reaction (HCR) was developed to detect LPS. Briefly, two complementary stable species of biotinylated DNA hairpins coexisted in solution until the introduction of a detection probe triggered a hybridization chain reaction cascade. The DNA conjugates specifically reacted with the LPS, which were captured by the ethanolamine aptamer attached to the reaction well surface. After optimizing the key reaction conditions, such as the reaction time of HCR, the amount of the capture probe and detection probes, the increase in the LPS concentration was readily measured by the optical density value and a relatively low detection limit (1.73 ng/mL) was obtained, with a linear response range of 1–105 ng/mL. The approach presented herein introduced the use of an aptasensor for LPS discrimination and HCR for signal amplification, offering a promising option for detecting LPS.
Similar content being viewed by others
Introduction
Lipopolysaccharides (LPS), also known as endotoxins, are integral components of the outer membrane of all gram-negative bacteria and consist of the following three distinct regions: O-specific antigen, core polysaccharide and lipid A1,2,3. The core polysaccharide, the stable component of all types of LPS, consists of two or more units of 2-keto-3-deoxyoctonic acid (KDO) linked to two or three L-glycero-D-manno-heptose (Hep), which are only present in bacteria4. The hydroxyl group in Hep and KDO is often replaced by ethanolamine5. Because LPS is responsible for pyrogenic reaction, septic shock, diarrhea, hypotension and vascular blood clotting, the detection of LPS is essential for medical, pharmacological and food safety6.
In the European and Chinese pharmacopoeia, the standard LPS detection method is the Limulus amebocyte lysate (LAL) assay7. LAL is based on the clotting reaction between Limulus and LPS, but usually takes several hours and false positive results can occur because Limulus can react with other LAL-reactive materials, such as β-(1,3)-D-glucan8,9,10. Silver staining is another popular LPS detection method. After silver staining, LPS can be separated by polyacrylamide gel electrophoresis11. Silver staining offers an inexpensive detection method using simple equipment and materials. Nonetheless, to visualize the gel-separated LPS, potentially hazardous formaldehyde must still be used, as it is an indispensable silver reductant12.
Recently, aptamers have been used in the detection of LPS13,14. Aptamers, artificial DNA/RNA oligonucleotides selected in vitro by SELEX, can fold into well-ordered, three-dimensional molecular architectures in which the ligand becomes an intrinsic part of the nucleic acid structure15. Compared to LAL and silver staining, aptamers have the obvious advantages of high affinity, high specificity, chemical stability, strong adaptability and stability in the detection environment. Su et al., reported an aptamer method for LPS detection that combined a gold nanoparticle/PEDOT (polymerized self-assembled monolayer of thiol-functionalized 3,4-ethylenedioxythiophene derivative, PEDOT) platform, which indicates that this method need more requirements for the detection environment16.
To increase the sensitivity of target analysis, researchers have introduced signal amplification systems11,17,18. Isothermal amplification technologies that have been used as effective tools to amplify detection signals include rolling circle amplification (RCA)19,20,21 and rapid isothermal detection and amplification (RIDA)22,23. These methods utilize a primer to extend an oligomer that is attached to the detection target. The extended DNA can then hybridize with multiple oligonucleotides bearing fluorescent probes and thus improve detection limits. However, both technologies rely on isothermal polymerase24. Hybridization chain reaction (HCR), which was first proposed by Dirks and Pierce, uses a pair of complementary, kinetically trapped hairpin oligomers to propagate a chain reaction of hybridization events25. Compared to RCA and RIDA, the obvious advantage of HCR is that it can amplify at room temperature without enzymes.
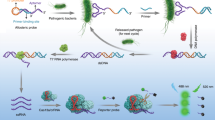
Herein, we developed a novel assay that combined HCR for signal amplification with an aptasensor for the detection of LPS using LPS from E. coli O111:B4 as the model analyte. In detail, biotinylated monomer DNA building blocks were mixed together but did not hybridize on an experimental time scale. Exposure to the detection probe opened the hairpins in the solution and triggered a chain reaction of hybridization events. Afterwards, streptavidin horseradish peroxidase (SA-HRP) was introduced to react with the biotinylated HCR DNA conjugates. Then, the HRP-DNA conjugates were added to 96-well plates where the LPS were captured by the immobilized capture probe and were washed by washing buffer. A visible optical signal only appeared in the presence of the target LPS. Our results showed that HCR for amplification combined with a double-aptamer sandwich method achieved better visual detection of LPS. Compared with traditional assays, this method provides a novel assay for simple, fast and highly sensitive detection in the field of monitoring LPS.
Materials and Methods
Materials and reagents
All chemicals were of analytical reagent grade and used as received. Water was purified by a Millipore Milli-XQ system (Millipore, Bedford, MA, USA). The DNA marker was purchased from Tiangen Biotechnology Co., Ltd (Beijing, China). LPS from E. coli O111:B4 and LPS from E. coli O55:B5 were purchased from Sigma-Aldrich Co., Ltd (Shanghai, China). Bovine serum albumin (BSA), streptavidin, tetramethylbenzidine (TMB) and SA-HRP were purchased from Beijing Biyuntian Co., Ltd (Beijing, China). Carboxyl-modified Nunc 96-well plates were purchased from Thermo Fisher Scientific Inc. (Rochester, NY, USA). The human serum samples were purchased from Food safety Technology Co., Ltd (Beijing, China). The nucleic acid sequences (5′-3′) used in this paper26,27,28 were in the Table 1. All oligonucleotides were synthesized by Life Biotechnology Co., Ltd (Beijing, China) and used as received after dissolving in water.
Apparatus
Visual measurements were carried out on a portable spectrophotometer (NS810, Shenzhen 3nh Technology Co., Ltd). The electrophoresis system consisted of a DYCP-31E vertical electrophoresis tank (Beijing Liuyi Instrument Plant, China) and a steady voltage electrophoresis power supply (PowerPacTM HV, Bio-Rad, USA). Images were recorded by the Gel Doc UV system (Bio-Rad, USA).
Gel electrophoresis
The target LPS was incubated with 200 nM aptamer consisting of 500 nM H1 and 500 nM H2 in 20 μL of reaction buffer (2.5 mM NaH2PO4, 8 mM Na2HPO4, 0.15 M NaCl, 2 mM MgCl2, pH 7.4) for 1 h at 37 °C. The agarose gel electrophoresis was run at 130 V for 30 min and then photographed with a digital camera.
Immobilization of the capture probe
First, 100 μL streptavidin (2 μg/L) in coating buffer (38 mM NaHCO3, 15 mM Na2CO3, pH 9.6) was added to the wells and incubated for 1 h at 37 °C. Then, the wells were washed three times with 150 μL of wash buffer (0.15 M NaCl, 10 mM K2HPO4, 1 mM NaH2PO4, 0.05% Tween 20, pH 8) to remove free streptavidin. Next, the samples were blocked with 100 μL of 10% BSA per well at 37 °C for 1 h to minimize non-specific adsorption and 100 μL biotinylated ethanolamine aptamer solution (100 nM) was added to wells and incubated at 37 °C for 1 h.
Extension of oligonucleotide chains by HCR
The aptamer and the hairpin oligonucleotides were heated to 95 °C for 2 min and then cooled to room temperature for 1 h before use. A 200 nM LPS aptamer was mixed with 500 nM H1 and 500 nM H2 in 5 mL of reaction buffer (8 mM Na2HPO4, 2.5 mM NaH2PO4, 0.15 M NaCl, 2 mM MgCl2, pH 7.4) and incubated for 37 °C for 1 h. Afterwards, 5 μL SA-HRP was added to the buffer and shaken at 37 °C for 1 h.
Peroxidase-Linked Microplate Procedure
After immobilization of the capture probe, 100 μL LPS was added to the wells and reacted at 37 °C for 1 h. The wells were then washed with wash buffer three times to remove free LPS. Next, 50 μL of the prepared HRP-DNA conjugates and 50 μL reaction buffers were added to the wells and incubated for 1 h at 37 °C. The resultant HRP conjugates were washed three times with 150 μL of wash buffer and then, 100 μL TMB peroxidase substrate was added to the wells to trigger the chromogenic reaction. Add 50 μL 2 M H2SO4 to the wells after 15 minute for terminating the reaction.
Results and Discussion
The principle of the HCR aptasensor
Non-enzymatically amplified visual measurements based on the aptasensor were used to identify and detect LPS at low concentrations. Scheme 1 illustrates the reaction scheme for the amplified detection of LPS from E. coli O111:B4. As shown in Fig. 1, the experiments can be divided into the following three parts: the immobilization of the capture probe, the HCR of the detection aptamer and peroxidase-linked microplate procedure. The first two reactions were carried out at the same time.
To immobilize the capture probe, the streptavidin was incubated in the wells. The biotinylated ethanolamine aptamer was bound in the well by the combination of streptavidin and biotin. In the HCR of the detection aptamer, the two hairpins could not open and hybridize with each other at 37 °C. The introduction of an detection probe strand triggered a chain reaction of alternating kinetic escapes by the two hairpin species into a nicked double helix. In this process, the amplification of the initiator recognition event continued until the supply of H1 or H2 was exhausted. The driving force for this reaction was the formation of polymers triggered by the aptamer and the alternating hybridization between the two hairpins. Because the two fuels were modified with biotin, we used SA-HRP to combine the HCR conjugates to achieve better visual detection. To maximize the hybridization efficiency, HCR was conducted in the solution instead of on a solid surface. Here, we achieved HRP-DNA conjugates as the detection aptamer. When the LPS from E. coli O111:B4 appeared, the ethanolamine aptamer captured them in the wells. After incubating with HRP-DNA conjugates and washing three times to remove the free LPS, we introduced TMB and H2O2, which can be reacted under HRP catalysis. The OD values were detected by a portable spectrophotometer.
Optimization of streptavidin and capture probe
The amount of streptavidin coating the wells plays an important role in the successful immobilization of the capture probe. According to Fig. 2A, the optical density value increased until the streptavidin coating was 2 μg/mL and then decreased as the streptavidin coating increased further. The decrease in the optical density value could be attributed to the saturation of the streptavidin coating on the wells29,30. Excess streptavidin coating would cause steric hindrance, reducing the binding with biotinylated capture probe.
(A) The effect of the streptavidin coating. Experimental conditions: the capture probe, LPS, the detection probe, H1/H2 and SA-HRP were 100 nM, 10 μg/mL, 500 nM/500 nM and 500 ng/mL, respectively. (B) The effect of the capture probe: the streptavidin coating, LPS, the detection probe, H1/H2 and SA-HRP were 2 μg/mL, 10 μg/mL, 100 nM, 500 nM/500 nM and 500 ng/mL, respectively. (C) The effects of detection probe sequence. Experimental conditions: the streptavidin coating, the capture probe, LPS and SA-HRP were 2 μg/mL, 100 nM, 5 μg/mL and 500 ng/mL, respectively. (D) The effects of SA-HRP. Experimental conditions: the streptavidin coating, the capture probe, LPS, the detection probe and H1/H2 were 2 μg/mL, 100 nM, 0.5 μg/mL, 50 nM and 250 nM/250 nM.
A range of quantities of ethanolamine aptamer were investigated as shown in Fig. 2B. The optical density value was initially observed to increase with the increasing concentration of the capture probe, reaching a maximum at 100 nM. Further increase in the concentration of the capture probe to 400 nM caused a slight decrease in the optical density value. Thus, 100 nM of the capture probe was selected for subsequent experiments.
Optimization of HCR
To confirm the hybridization chain reaction, native gel electrophoresis was performed revealing a distribution of polymer lengths. Fig. S1 demonstrates that the hairpins could only polymerize in the presence of the detection probe and the target LPS did not affect the hybridization of three strands.
The effects of the amount of the detection probe sequence, the concentration of SA-HRP, the HCR reaction time and the reaction temperature were subsequently examined and optimized.
The optical density value increased as the amount of detection probe was increased from 10–50 nM and then decreased slightly as the amount was further increased (Fig. 2C). Thus, 50 nM of the detection probe was selected for subsequent experiments. The optical density value also increased with the concentration of SA-HRP (125–500 ng/mL) and then remained stable with further increases in the SA-HRP concentration (Fig. 2D). Therefore, 500 ng/mL SA-HRP was selected for use in further studies.
The time spent is an important indicator to evaluate the merits of aptasensors. We could clearly see that for the HCR polymerization, the number of DNA conjunctions increased from 0.5 h to 1 h and then remained almost constant (Fig. 3A). It means until the supply of H1 or H2 was exhausted after 1 h, the amplification of the initiator recognition could complete for 1 h. In order to improve detection efficiency of the aptasensor, we chose 1 h as the HCR. polymerization time for detection. The reaction temperature was critical for detection. We selected 10 °C, 15 °C, 25 °C and 37 °C as the reaction temperatures and HCR was triggered at all of these temperatures (Fig. 3B). However, slight diffusion strips appeared in the electrophoresis’s 19–20 rows. There were DNA duplexes whose molecular weight were >150 bp even >500 bp. And DNA duplexes were same with the product of HCR. So we inferred that H1 and H2 could be triggered without the initiator at 15 °C and this temperature should not be used as the reaction temperature.
(A) The effects of reaction time. Experimental conditions: (1–10) the detection probe andH1/H2 were 50 nM and 250 nM/250 nM, respectively. (1, 2) reaction time was 0.5 h, (3, 4) reaction time was 1 h, (5, 6) reaction time was 2 h, (7, 8) reaction time was 3 h, (9, 10) reaction time was 4 h; (11, 12) 50 nM of the detection probe; (13, 14) 250 nM of H1; (15, 16) 250 nM of H2; (17, 18) 250 nM of H1 and 250 nM of H2, reaction time was 4 h. (B) The effects of reaction temperature. Experimental conditions: (1, 2) 50 nM of the detection probe; (3, 4) 250 nM of H1; (5, 6) 250 nM of H2; (7, 8, 11, 12, 15, 16, 19, 20) 250 nM of H1 and 250 nM of H2; (9, 10, 13, 14, 17, 18, 21, 22) the detection probe and H1/H2, were 50 nM, 250 nM/250 nM. (7–10) reaction temperature was 37 °C, (11–14) reaction temperature was 25 °C, (15–18) reaction temperature was 15 °C, (19–22) reaction temperature was 10 °C.
Amplification effect of HCR
To prove the amplification effect of HCR, we compared the HCR aptasensor and a non-HCR aptasensor, which employed a biotinylated aptamer of the LPS from E. coli O111:B4. As shown in Fig. 4, the optical density value of the HCR-based system was obviously higher than that of the non-HCR system and the sensitivity was noticeably improved, clearly indicating that the optical density value incorporated in the HCR process was at least 4-fold greater than that in the non-HCR method.
The optical density value vs. different detection methods.
Experimental conditions of HCR: capture probe, detection probe, H1/H2 and SA-HRP were 100 nM, 50 nM, 250 nM/250 nM and 500 ng/mL, respectively; Experimental conditions of Non-HCR: capture probe, detection probe and SA-HRP were 100 nM, 50 nM and 500 ng/mL, respectively; the reaction temperature was 37 °C. The detection procedure was carried out as described in the Experimental section.
Assay performance
Sensitivity and specificity are two important parameters for a successful LPS assay system. In this study, the sensitivity was primarily dependent on the formation of polymerizing hairpins. The detection specificity was basically determined by the function of the capture probe and detection probe. To evaluate the detection specificity of the aptasensor, D-mannose, BSA, peptidoglycan from Staphylococcus aureus and LPS from E. coli O55:B5 were chosen as the non-specific species and investigated. As shown in Fig. 5, high optical density values were only obtained in the presence of LPS from E. coli O111:B4. The optical density values were weak in the presence of D-mannose, BSA, peptidoglycan from Staphylococcus aureus or LPS from E. coli O55:B5 at 500 ng/mL and were comparable to the background signal. These data demonstrated that the optical density value was specifically high for the aptamer/target binding, which indicated that the aptasensor we proposed here exhibited good performance for discriminating LPS from E. coli O111:B4 from other interfering bindings.
Quantification of LPS
Examination of the relationship between optical density value and the amount of LPS revealed a linear relationship in the concentration range of 1–105 ng/mL (Fig. 6), represented by y = 0.1549ln(x) − 0.0751 (R2 = 0.9891). The LOD is 1.73 ng/mL, calculated by the standard formulae, as mentioned below and further specified in the literature31,32,33. Each data point was the average of three individual measurements.

where OD.LOD is the optical density corresponding to LOD, respectively; OD.Blank is the optical density of the blank; and SD.Min Analyte Conc. is the standard deviations of the minimum analyte concentration, respectively.
Assay of LPS in drinking water and human serum samples
To evaluate the detection application of this colorimetric method, we carried out a recovery test of LPS in drinking water and human serum. Different concentrations of LPS were added into drinking water and 5-fold diluted human serum samples. The recoveries of LPS detected in the drinking water samples ranged from 97.00% to 103.83% (Table S1). The recoveries of LPS detected in the serum samples ranged from 98.23% to 102.00% (Table S2). These results were satisfactory for quantitative assays performed in biological samples.
Conclusions
In this paper, a novel HCR-based aptasensor was developed for the highly sensitive detection of LPS. In our design, two kinds of aptamers were introduced which could bind the LPS with high affinity and high specificity. HCR was employed as the amplification tool. A quantitative analysis of target LPS was then carried out by measuring the optical density value. The relatively low detection limit was 1.73 ng/mL, with a linear response range of 1–105 ng/mL. Compared with other methods of LPS detection34, our aptasensor based on HCR provides a visual and method with the same detection limitation for the highly sensitive detection of target LPS. In practical applications, the detection probe and the 96 wells which were coated by the capture probe can be prepared in advance, so they can detect the target LPS in less time. This novel technique is isothermal and can easily be adopted for use as an aptasensor for molecular detection.
Additional Information
How to cite this article: Xie, P. et al. Highly sensitive detection of lipopolysaccharides using an aptasensor based on hybridization chain reaction. Sci. Rep. 6, 29524; doi: 10.1038/srep29524 (2016).
References
Varbanets, L. D., Zdorovenko, E. A., Garkavaya, E. G. & Brovarskaya, O. S. Lipopolysaccharide of Escherichia coli M-17. Microbiology 81, 324–331 (2012).
Pieretti, G., Carillo, S., Kim, K. K. et al. O-chain structure from the lipopolysaccharide of the human pathogen Halomonas stevensii strain S18214. Carbohydrate research 346(2), 362–365 (2011).
Otto, H. The structures of core regions from enterobacterial lipopolysaccharides - an update. FEMS Microbiology Letters 271, 3–11 (2007).
Drago, S. et al. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. International Immunopharmacology 12, 1–9 (2012).
Lodowska, J., Wolny, D., Weglarz, L. & Dzierzewicz, Z. Postepy Higieny I Medycyny Doswiadczalnej 61, 106–121 (2007).
Anje, C., Elke, R., Ben, J. & Peter, B. Reactive oxygen species and small-conductance calcium-dependent potassium channels are key mediators of inflammation-induced hypotension and shock. Journal of Molecular Medicine 9, 921–930 (2010).
Pedersen, M. R., Hansen, E. W. & Christensen, J. D. Detection of lipoplysaccharide in the picogram range of tissue culture media by a kinetic chromogenic limulus amebocyte lysate assay. Journal of Clinical Pharmacy and Therapeutics 19, 189–194 (1994).
Mark, F., Jonathan, P., Joanne, B. & Miller, J. D. Modification of the Limulus amebocyte lysate assay for the analysis of glucan in indoor environments. Analytical and Bioanalytical Chemistry 379, 156–162 (2004).
Alwis, K. U. & Milton, D. K. Recombinant factor C assay for measuring endotoxin in house dust: Comparison with LAL and (1→ 3)‐β‐D‐glucans. American journal of industrial medicine 49(4), 296–300 (2006).
Yao, M. et al. Comparison of electrostatic collection and liquid impinging methods when collecting airborne house dust allergens, endotoxin and (1, 3)-β-d-glucans. Journal of Aerosol Science 40, 492–502 (2009).
Qiang, W. et al. A fluorescent biosensing platform based on the polydopamine nanospheres intergrating with Exonuclease III-assisted target recycling amplification. Biosensors and Bioelectronics 71, 143–149 (2015).
Zhu, Z. et al. An improved silver stain for the visualization of lipopolysaccharides on polyacrylamide gels. Electrophoresis 33, 1220–1223 (2012).
Wu, W., Zhang, J., Zheng, M. et al. An aptamer-based biosensor for colorimetric detection of Escherichia coli O157: H7. PloS one 7(11), e48999 (2012).
Zuo, M. et al. Detecting endotoxin with a flow cytometry-based magnetic aptasensor. Analytical Biochemistry 466, 38–43 (2014).
Kandimalla, V. B. & Huang, X. J. New horizons with a multi dimensional tool for applications in analytical chemistry - Aptamer Analytical Letters 37, 2215–2233 (2014).
Su, W. et al. Aptamer‐Assisted Gold Nanoparticles/PEDOT Platform for Ultrasensitive Detection of LPS. Electroanalysis 25, 380–386 (2013).
Zhang, L. Q. et al. A new system for the amplification of biological signals: RecA and complimentary single strand DNA probes on a leaky surface acoustic wave biosensor. Biosensors and Bioelectronics 60, 259–264 (2014).
Sang, H. S. et al. Highly sensitive detection of a bio-threat pathogen by gold nanoparticle-based oligonucleotide-linked immunosorbent assay. Biosensors and Bioelectronics 64, 69–73 (2015).
Yao, L. et al. Integrated platform with magnetic purification and rolling circular amplification for sensitive fluorescent detection of ochratoxin A. Biosens Bioelectron 74, 534–538 (2015).
Gao, F. et al. A cascade signal amplification strategy for surface enhanced Raman spectroscopy detection of thrombin based on DNAzyme assistant DNA recycling and rolling circle amplification. Biosensors and Bioelectronics 66, 423–430 (2015).
Chen, H. et al. Detection of vascular endothelial growth factor based on rolling circle amplification as a means of signal enhancement in surface plasmon resonance. Biosensors and Bioelectronics 61, 83–87 (2014).
Zheleznaya, L. A. Perevyazov, T. A. Zheleznyakov, E. N. & Matvienko, N. I. Some properties of site-specific nickase BspD6I and the possibility of its use in hybridization analysis of DNA. Biochemistry 67, 498–502 (2002).
Gao, W. et al. Rapid isothermal detection assay: a probe amplification method for the detection of nucleic acids. Diagnostic microbiology and infectious disease 60, 133–141 (2008).
Appleyard, D. C., Chapin, S. C. & Doyle, P. S. Multiplexed protein quantification with barcoded hydrogel microparticles. Analytical Chemistry 83, 193–199 (2011).
Dirks, R. M. & Pierce, N. A. Triggered amplification by hybridization chain reaction. Proceedings of the National Academy of Science of the United States of America 101, 15275–15278 (2004).
Song, W., Zhu, K., Cao, Z. et al. Hybridization chain reaction-based aptameric system for the highly selective and sensitive detection of protein. Analyst 137(6), 1396–1401 (2012).
Bruno, J. G., Carrillo, M. P. & Phillips, T. In vitro antibacterial effects of antilipopolysaccharide DNA aptamer-C1qrs complexes. Folia microbiologica 53(4), 295–302 (2008).
Mann, D., Reinemann, C., Stoltenburg, R. et al. In vitro selection of DNA aptamers binding ethanolamine. Biochemical and Biophysical Research Communications 338, 1928–1934 (2005).
Bouças, R. I. et al. Development of an enzyme-linked immunosorbent assay (ELISA)-like fluorescence assay to investigate the interactions of glycosaminoglycans to cells. Analytica Chimica Acta 618, 218–226 (2008).
Lanyon, H. T. et al. Click-Chemistry Armed Enzyme Linked Immunosorbent Assay to Measure Palmitoylation by Hedgehog Acyltransferase. Anal Biochem. 490, 66–72 (2015).
Sandeep, K. V., Schneider, E. M., Edmond, L., Sabahudin, H. J. & Luong, H. T. One-step antibody immobilization-based rapid and highly-sensitive sandwich ELISA procedure for potential in vitro diagnostics. Scientific Reports 4, 4407 (2014).
Dixit, C. K., Vashist, S. K., MacCraith, B. D. & O’Kennedy, R. Multisubstratecompatible ELISA procedures for rapid and high-sensitivity immunoassays. Nat. Protoc. 6, 439–445 (2011).
Vashist, S. K., Saraswat, M. & Holthofer, H. Comparative study of the developed chemiluminescent, ELISA and SPR immunoassay formats for the highly sensitive detection of human albumin. Procedia Chem. 6, 184–193 (2012).
Iijima, S. et al. Enzymatically amplified electrochemical detection for lipopolysaccharide using ferrocene-attached polymyxin B and its analogue. Biosensors and Bioelectronics 26, 2080–2084 (2011).
Acknowledgements
This work was supported by the Beijing New-star Plan of Science and Technology xx2014B069. Funding for open access charge: Beijing Academy of Science and Technology.
Author information
Authors and Affiliations
Contributions
P.X., W.X. and K.H. conceived and designed the experiments. P.X., L.Z. and X.S. performed the experiments. P.X., L.Z. and J.T. analyzed the data. P.X. wrote the paper. W.X. and K.H. rivised the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, P., Zhu, L., Shao, X. et al. Highly sensitive detection of lipopolysaccharides using an aptasensor based on hybridization chain reaction. Sci Rep 6, 29524 (2016). https://doi.org/10.1038/srep29524
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29524
This article is cited by
-
Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease
Nature Reviews Cardiology (2023)
-
Ultrasensitive colorimetric detection of circulating tumor DNA using hybridization chain reaction and the pivot of triplex DNA
Scientific Reports (2017)
-
Detection of endotoxins using nanomaterials
Toxicology and Environmental Health Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.