Abstract
Predicting fish responses to modified flow regimes is becoming central to fisheries management. In this study we present an agent-based model (ABM) to predict the growth and distribution of young-of-the-year (YOY) and one-year-old (1+) Atlantic salmon and brown trout in response to flow change during summer. A field study of a real population during both natural and low flow conditions provided the simulation environment and validation patterns. Virtual fish were realistic both in terms of bioenergetics and feeding. We tested alternative movement rules to replicate observed patterns of body mass, growth rates, stretch distribution and patch occupancy patterns. Notably, there was no calibration of the model. Virtual fish prioritising consumption rates before predator avoidance replicated observed growth and distribution patterns better than a purely maximising consumption rule. Stream conditions of low predation and harsh winters provide ecological justification for the selection of this behaviour during summer months. Overall, the model was able to predict distribution and growth patterns well across both natural and low flow regimes. The model can be used to support management of salmonids by predicting population responses to predicted flow impacts and associated habitat change.
Similar content being viewed by others
Introduction
Modified flow regimes can impact valuable freshwater fisheries and are likely to become an increasing threat due to greater water extraction, flow regulation and climate change driven modified temperature and precipitation patterns1,2,3. English chalk stream conditions of high invertebrate densities, low turbidity and favourable summer temperatures make them prime habitat for brown trout (Salmo trutta) and Atlantic salmon (Salmo salar)4,5,6 but their ecology is threatened by modified flows7,8. In the past, habitat association models have been commonly used to predict fish population responses to environmental change9; however, they are limited in their use in conditions outside of the ones for which they were parameterised10. Furthermore, this modelling approach does not account for fish behaviour as an adaptive response to an environmental change such as modified flow11. There is a need to pursue models capable of including an adaptive response in order to increase confidence in predicting fish population responses to habitat and support an evidence-based management approach12,13,14.
In fisheries management, two important population patterns are 1) how much fish grow and 2) how they distribute themselves across habitats. In temperate systems, fish growth is often greatest in productive summer months and can drive overwinter fish survival rates of fish whilst underpinning management stocking decisions15,16. In a modified flow environment, reduced flows can affect drift-feeding fish consumption rates and thus growth by altering flow velocities, depth and resource densities17. However, salmonids are territorial18,19 and individual fish moving in response to reduced flow will be limited by the availability of suitable areas for feeding20,21. The inclusion of behavioural processes is important for robust predictive models, as habitat and population patterns are driven by animal behaviour11,22. Models should also have the capacity to produce temporal and spatial site-specific population predictions.
One approach capable of linking behaviour to individual, population and environmental processes is individual-based ecology and can be implemented as Agent-Based/Individual-Based Models (ABM)23. MORPH is an ABM platform operating on optimal-foraging and fitness maximising principles; virtual ‘animals’ adapt their behaviours to feed or to rest based on bioenergetic rules, within virtual environments24 and has been successfully applied to coastal bird systems25,26. Elsewhere, non-MORPH salmonid ABMs have been developed and applied for fish populations in North America27.
In this study, we adapted the original MORPH ABM and assessed the ability of the model to predict salmonid somatic growth and distribution responses to flow changes. First, we recorded the environmental conditions and population patterns of a wild chalk stream salmonid population under natural flow (NF) and then modified, low-flow conditions (MLF). We then parameterised the agents in FishMORPH to have salmonid drift-feeding behaviour and bioenergetics using models obtained from literature. Appropriate habitat selection behaviours are needed to produce realistic patterns in spatially-explicit ABMs28 and we compared model results from alternative habitat selection models against the somatic growth and distribution of real salmonids under the two flows observed from the field study. The virtual fish habitat selection models tested were 1) random, 2) maximises consumption rates (MCR), 3) minimises predator-prey ratio (MPR), or 4) prioritises consumption rates over predator:prey ratios (CR > PR). The behaviour that most closely replicated the observed patterns was considered the most appropriate to be used in FishMORPH following a Pattern-Orientated Modelling approach (POM)29.
Results
In this section we first describe the field study site and report the data collected from it. We then report the results comparing the growth and distribution from fish in the field study against the same pattern from virtual fish following the different movement rules.
Field study
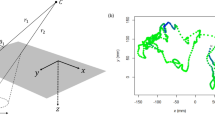
Our study site was a section (520 m length; 6.25 m mean width) of the chalk stream part of the river Frome, United-Kingdom (50° 40′44″N; 2° 0′42″ W). We classified it at two spatial resolutions i) stretches (circa. 80 m in length) and ii) patches (39.6 ± 14.15 m2, mean ± SE) (Fig. 1). No barriers prevented fish movement. The study period began with NF conditions (17 July–23 September 2008, 68 days) after which we closed upstream river hatches to create MLF conditions (23 September–10 October 2008, 17 days). We recorded i) water temperature, ii) discharge and iii) daylight hours, and applied these to the entire study site as ‘global’ parameters in the model (see Supplementary Table S1). We also recorded i) predator densities and collected data on invertebrate densities to estimate ii) invertebrate prey abundance and applied these as stretch-specific parameters in the model (see Supplementary Table S2).
A schematic map of the study site, a section (520 m length; 6.25 m mean width) of chalk stream part of the River Frome, United-Kingdom (50° 40′44″N; 2° 0′42″ W) showing the two spatial resolutions i) 7 stretches (solid lines, large numbers, circa. 80 m in length) and the ii) 75 patches (dotted lines, small numbers, 39.6 ± 14.15 m2, mean ± SE).
We measured body mass and specific growth rates (SGR) from four electric fishing surveys. We caught a total of 1453 YOY Atlantic salmon, 283 YOY and 81 1+ brown trout of which 94, 96, and 79 were marked recaptures respectively. Over both flows, mean fish body mass increased and there was greater population variation in SGR during the MLF period (Fig. 2). YOY Atlantic salmon SGR showed the greatest pattern of change from the NF to MLF period while more YOY Atlantic salmon recorded slower growth rates in the latter period (Fig. 2).
The fish were young-of-the-year (YOY) and one-year-old (1+) Atlantic salmon (Salmo salar) and brown trout (Salmo trutta). Plots in the left hand column show body mass and right hand for specific growth rates. For the body mass, each data point for field study results is an individual fish and model boxplots are from five replicate simulations. The white background indicates the period of natural flow and the grey background indicate the period of modified low flow. For specific growth rates (SGR), each data point for field study results is an individual recaptured PIT tagged fish and boxplots are of tagged fish in model from five replicate simulations. The stars indicate if the 95% quartile of differences between the movement rule model predicted and observed SGR from Bayesian statistics does not include zero.
Validation of model predicted patterns
We then used the observed spatial and temporal patterns from the field study as the point of comparison for patterns generated from simulations of virtual fish following the four movement rules to validate the model.
Pattern replication from 1) random and 3) MPR movement rules
Virtual fish that moved either randomly or MPR did not grow but consistently lost weight and had final distributions of very low body mass. These were not at all similar to observed body mass distribution patterns collected from field work and we removed randomly and MPR models from further analysis following the POM approach29.
Body mass and specific growth rates pattern replication by 2) MCR and 4) CR>PR movement rules
We compared the body masses of MCR and CR>PR virtual fish against recorded body masses of real fish at time steps corresponding to when we performed fish surveys during the field study and found they were very similar across all three fish groups (Fig. 2). Both models produced similar median SGRs for each fish category and matched median observed SGRs well except for 1+ brown trout, where model SGRs were greater than those observed. Bayesian estimation of the probability of the difference between mean growth rates show that observed and virtual fish matched well for YOY brown trout across both flow periods, while YOY Atlantic salmon SGRs matched the NF period but not the MLF period (Fig. 2 and see Supplementary Table S3). Additionally, the model under predicted the observed variation in SGR in all cases.
Stretch distribution pattern replication by 2) MCR and 4) CR>PR movement rules
We compared the stretch distributions predicted by CR>PR and MCR virtual fish with observed distributions30 at time points corresponding to when we performed the fish surveys during the field study and found they matched the observed distribution well across both flow regimes (Fig. 3). When pattern matching was analysed over both flow periods, CR>PR produced stretch distributions with higher Pearson’s r for YOY Atlantic salmon and YOY brown trout but not for 1+ brown trout, where MCR fish distributions were higher.
Each point represents the mean and standard error of a stretch at 33, 68 (natural flow period) and 85 days (modified low flow) of the study period. Left-hand panels show data for the MCR models, right hand for CR>PR models. Rows correspond to fish categories as in Fig. 2.
Patch distribution pattern replication by 2) MCR and 4) CR>PR movement rules
We compared the patch occupancy distribution predicted by CR>PR and MCR virtual fish with observed distributions at time points corresponding to when the surveys were performed and found they were visually very similar (Fig. 4). In the patches where we tracked high numbers of fish the predicted patch densities are higher and vice versa. The Mean Absolute Error (MAE) between the observed and predicted was lower for the CR>PR movement rule for all distributions except for YOY Atlantic salmon MLF period. The difference between the two movement rules was greatest for 1+ brown trout. The fewer fish in the observed pattern was due to either tracking inefficiencies (estimated at ~40–50%31), tag loss or death by fish in the real study. However, the high number of sampling surveys (18 and 7 PIT tag tracking surveys during the NF and MLF periods respectively) provided a fairly robust overall pattern of the patches occupied or not occupied by fish.
Each bar point corresponds to one patch from 18 and 7 PIT tag tracking surveys during the natural flow and modified low periods. The bars show the mean and standard error from five replicate simulations at time steps corresponding to when the tracking surveys were performed in the field study. Gaps in the observed pattern indicate no fish were tracked in the patch in any of the surveys. Model standard error bars were obtained from five replicate simulations. White bars show the observed numbers and black bars are model predicted data. Left-hand panels show data for the natural flow period, right hand for the period of modified low flow. Rows correspond to fish categories as in Fig. 2.
Proportion of time spent feeding predicited under 2) MCR and 4) CR>PR movement rules
We analysed the amount of time virtual fish moving under MCR and CR>PR movement rules spent feeding and found MCR fish fed for less time than CR>PR fish suggesting they reached the model defined time step maximum consumption threshold quicker (Fig. 5). Virtual fish feeding for the whole time step were likely not to reaching consumption maximum and so their overall growth rate would likely not be as high. The proportion of time spent feeding increased in the MLF period but the majority of fish still reached the threshold (i.e. less than 100% of the time spend feeding).
Each box plot with outliers is the combined distribution of individual fish in five replicate simulations and time steps correspond to when tracking surveys were performed in Fig. 3. The white background indicates the period of natural flow and the grey background indicates the period of modified low flow. Left-hand panels show data for the MCR models, right hand for CR>PR models. Rows correspond to fish categories as in Fig. 2.
Sensitivity analysis
Sensitivity analysis showed the model was most sensitive to parameters associated with bioenergetics whilst parameters pertaining to behavioural drift-feeding submodels had smaller effects (see Supplementary Table S4).
Discussion
Robust predictions of the complex salmonid ecological response to altered flow regimes are needed for a proactive, evidence-based approach to fishery management. FishMORPH was able to predict body mass, growth rates (Fig. 2) and stretch and patch distribution (Figs 3 and 4) patterns of YOY and 1+ chalk stream Atlantic salmon and brown trout across two contrasting flow regimes. There was no calibration of unknown parameters and all patterns were independent from model construction: this strengthens the credibility of the model. The inclusion of adaptive behaviours increases predictive realism over other models where it is omitted. The CR>PR rule produced patterns most similar to the observed patterns, closely followed by the MCR movement rule, which performed less well in predicting distribution. The consistency in replicating distribution patterns across the two flow regimes supports the assumption of salmonid behavioural movement consistency across contrasting flows.
In our model, movement rules based solely on minimising predator:prey ratios (MPR) did not replicate patterns as well as consumption prioritising rules (MCR and CR>PR). The primary growth period for chalk stream salmonids is during the high productivity and thermally optimal summer and early autumn months and fish must maximise the potential growth for increased overwinter survival32,33 and reproductive success34,35. The lack of support for predator orientated movement rules may be due to low predation pressure at the study site. Whilst the density of the main piscivorous predator, European pike, in this study was not managed and can be considered natural, Atlantic salmon and brown trout are not preferred pike prey36 and other fish species were present. In addition, interactions in chalk streams may be limited for YOY salmonids as they associate with high-velocity habitats and ambush predators, such as pike, prefer lentic ones37. Future simulations could test the capacity of a consumption rate prioritising movement rule to predict growth and distribution patterns in habitats with higher rates of predator interactions and be used to comment on predator culling management regimes where reduced densities of large predators lead to increased densities of small ones38. Indeed, more patterns can be included for further testing of model assumptions and refinement of movement rules28,39 and in habitats beyond those simulated in this study39. Random and MPR movement rules produced unrealistic growth patterns and were removed from consideration but while ecologically unrealistic, the simulations were useful for testing the model setup40.
The model closely predicted mean observed body mass and growth rates of YOY brown trout for both NF and MLF periods. It better predicted these patterns in YOY Atlantic salmon during the NF period than in the MLF period where the model predicted high growth while the observed rates showed a decline. It is likely that the model predicted growth rates to remain high because the recorded thermal regime during the MLF period supported maximum growth41 and potential food consumption was not a limiting factor in the model as virtual fish were still able to reach their maximum consumption threshold in this period (Fig. 5). However, the MLF period of the field study coincided with the onset of autumn and possible altered energy budgets for overwintering and possible autumn smolt migration for anadromous Atlantic salmon42,43. The smoltification process occurs over several months and is a significant physiological and behavioural transformation requiring energy and can lead to a reduction of body condition44,45. In this system, Atlantic salmon parr most commonly undergo a spring migration but a proportion will also undertake a downstream migration phase in the autumn42; the timing of the MLF period in this study would have also been the period when any autumn migrating Atlantic salmon parr would have begun migration. Bioenergetic processes associated with overwintering and smolting were not included in this current model and it remains to be seen if including a more complex bioenergetic model would improve FishMORPH’s replication of the observed YOY Atlantic salmon growth pattern in the MLF period. It is also unknown whether the decline in observed growth is due to low flow conditions or overwintering/smolting processes.
The model was able to predict the increase in population SGR variation from the NF to MLF period but overall predicted variation was smaller than in the observed data. This could be a result of low intra-individual and inter-cohort variation between virtual fish in the model. Intra-individual variation in virtual fish was found in parameters of starting body mass, processing order, territorial size and predator vulnerability. While variation in body mass (i.e. fish size) would lead to slightly different inputs into feeding (e.g. capture area) and bioenergetic (e.g. Cmax and Rmax) submodels, the submodels themselves were the same for every fish. In reality, fish are likely to have individual differences in metabolism that would result in different growth rates before feeding back and leading to variation in behavioural responses46. A model that includes possible inter-individual variation may improve the match between predicted and observed distribution variance but a compromise with model complexity was made47. Additionally, behavioural variation including the time and bioenergetic cost of aggressive behaviours48, nocturnal feeding switching, prey switching and reproduction may improve predictions for 1+ fish.
Sensitivity analysis identified that the model is most sensitive to bioenergetic parameters, which is likely due to the productivity of the chalk stream environment. Prey density is very high in these stream environments with densities of 170,000 invertebrates.m−2 having been recorded in chalk streams49. At these densities, prey is not likely to be a limiting factor in chalk streams; maximum consumption limits and/or access resulting from territorial behaviour are important predictors of salmonid population ecology21, but ultimately prey will become a limiting factor at sufficiently low densities. Indeed, there is a negative relationship between salmonid territory size and prey densities50, which will affect the relative sensitivity of the model to the territory size parameter along a prey density gradient. This dynamic relationship is not currently present in FishMORPH as territory size is a fixed constant per virtual fish age group.
IBM validation is most commonly performed through pattern-orientated modelling, as more common statistical, empirical techniques applied in non-modelling situations are not always relevant because of lack of completeness of input parameters and inappropriate statistical techniques51. The undertaking of a field study specifically designed for model parameterisation and pattern collection provided relevant input data. Bayesian techniques, as used here, are a promising avenue of statistical inferences to measure pattern reproduction and can be used in other areas of IBM construction (e.g. parameterisation and calibration52. Statistical techniques were used to complement the POM validation philosophy; we discarded Random and MPR movement rules because they were unable to replicate body mass and growth patterns.
Predictions from a validated ABM can be used to investigate theoretical and applied questions40,53 and FishMORPH is able to replicate growth and distribution patterns from a real salmonid population responding to a changing flow regime. It can be used to predict the population response to fairly basic management questions (e.g. increasing the number of virtual fish to identify optimal stocking densities on growth). FishMORPH can also be used to investigate more complex and interactive relationships (e.g. magnitude of reduced flow and thermal regime warming) and across scales by altering multiple parameters operating (e.g. individual fish bioenergetics and reduced food densities). Finally, validated ABMs like FishMORPH can help support fishery management decisions responding to altered flow regimes and facilitate discussions about managing water with other water-use sectors.
Methods
FishMORPH was parameterised and validated following a three-stage process; 1) a field study to collect input parameters and pattern data, 2) the construction of a bioenergetic and behaviourally realistic fish IBM within MORPH and 3) comparing predicted against observed patterns of growth and distribution (See Supplementary Figure S1).
Ethics statement
All animal procedures followed strict guidelines set forward by the Home Office, UK and were performed within accordance with UK Home Office Regulations. The project was approved by the Bournemouth University ethics committee and was performed under the Home Office project licence number PPL 30/2626.
Stage 1 – Field study
We recorded water temperature, discharge, daylight hours (sunrise and sunset), water height, channel gradient and aquatic vegetation patch cover across the study site (see Supplementary Methods). Drift and benthic invertebrate densities were collected from each stretch at dawn, midday and at dusk at monthly intervals, sorted into ten categories (1–3, 3–5, 5–7, 7–9 & 9–12 mm; aquatic or terrestrial in origin) and identified to family level. We accounted for the effect of clogging on drift estimation by drift net sampling54 by estimating drift density (invertebrates.m−3) from benthic invertebrate densities and drift net data to characterise its size distribution (see Supplementary Methods and Supplementary Table S5). There was a significant diel trend in drift densities in aquatically originating invertebrates (Kruskal-Wallis, p = 0.01) but not for terrestrial invertebrates (Kruskal-Wallis, p = 0.77). Thus, we parameterised the virtual environment to have aquatic invertebrates densities with intra-daily dynamics and terrestrial invertebrate densities that remained constant within a day. We found spatial and temporal differences in invertebrate densities across the study site and used linear interpolation between estimated densities to parameterise the invertebrate densities between sampling points (see Supplementary Methods).
We carried out a total of four fish surveys on 17th July, 19th August, 23rd September and 10th October 2008 (see31,55 and see Supplementary Methods). The number of Atlantic salmon and brown trout per stretch, fork length (FL, nearest mm), mass (M, nearest 0.1 g) and age (from scale samples) was recorded. A total of 91 YOY Atlantic salmon, 52 and 32 (YOY and 1+ respectively) brown trout were PIT tagged in the first survey. For each stretch and survey date, we estimated the number of fish and probability of capture56. Finally, we recorded the patch distribution of tagged fish by PIT-tag tracking surveys using a portable PIT detector31.
Stage 2 – Model construction, virtualisation of the environment and initial population setup
The IBM was implemented using MORPH, an optimal-foraging IBM modelling platform24. Model description follows the Overview, Design, Details (ODD) protocol57,58. The model code and associated parameter files are available online (see Supplementary Data). The model’s virtual environment was defined to closely match the recorded field study conditions. The initial virtual fish population matched the measured size and distribution of the real fish at the start of the field study.
Overview
Purpose
The aim was to model summer movement and growth of young-of-the-year (YOY) and one-year-old (1+) Atlantic salmon and brown trout under different discharge conditions using movement rules involving consumption rates and predator:prey ratio. Model validation was performed by comparing predicted patterns of body mass, growth and distribution against those collected from the field study.
Entities, state variables and scales
Simulations encompassed the NF and an MLF period of the field study and the model timestep was an hour. The virtual environment was a closed system with hierarchal spatial scales: i) global, ii) stretch and iii) patch. Global parameters applied across the entire environment with stretch and patch scales reflected parameters specific to their respective scales from the field study. Global variables were total duration (timesteps), day or night, water temperature (°C) and discharge (m3.s−1). The mass (dry mass, g) of invertebrates in the different invertebrate resource categories, their bioenergetic content (kJ.g−1) and the bioenergetic content of the fish (kJ.g−1) were kept constant (see Supplementary Table S6). Stretch variables were prey and predator densities (see Supplementary Table S2 and S5). Prey density (invertebrates.m−3) was defined into ten categories (1–3, 3–5, 5–7, 7–9 & 9–12 mm; aquatic or terrestrial in origin). Predator densities were modelled as a stretch parameter (no.m−2) and split into two size categories (small, FL<220 mm; large, FL>396 mm) to represent gape-limited predation on salmonids, no predators between the two thresholds were caught during the field study. Patch parameters were area (m2), location (in relation to other patches), the proportion of total area that is flowing (vs. slack water) (%), mean depth (m) and velocity (m.s−1) (see Supplementary Table S2).
Virtual fish were modelled as discrete individuals. Their constant state variables were species, age cohort, starting body mass, tagged status, starting stretch, territory size and diet. Fish diet was determined by fish length and prey-size diets. Dynamic state variables were current body mass (g) and location.
Variable processing and scheduling
FishMORPH processes variables in the order: 1) global, 2) stretch, 3) patch and 4) forager24 (see Supplementary Figure S2). Salmonid dominance is size-dependent20 and the model assumed size differences large enough to affect hierarchy were present between age cohorts but not within; 1+ fish were processed before YOY fish and fish in the same age cohort were processed in a random order.
Virtual fish had the following state variables parameterised: fork length (FL, mm), maximum consumption rate (Cmax, kJ.hr−1), maximum respiration rate (Rmax, kJ.hr−1), standard respiration rate (Rs, kJ.hr−1), respiration cost of digestion (Rd, kJ.hr−1), swimming cost whilst feeding (SCfeeding, kJ.hr−1), resting (SCresting, kJ.hr−1), reaction distance (RD, m), maximum swimming velocity (Vmax, m.s−1), and capture area (CA, m2). Further prey consumption parameters were also modelled: encounter rate (RE, prey.hr−1), capture probability success (CPS, %), and capture rate (CR, prey.hr−1). Within a time step, fish were modelled as feeding or resting (or both) and selected the proportion returning the highest growth. Fish could only feed if there was sufficient feeding space. Bioenergetic equations of consumption and expenditure are used to calculate fish body mass at the end of each time step. This was then used as an input for calculating the changes during the next time step (see Supplementary Figure S3).
Design concepts
The model’s basic principle was optimal-foraging theory24 with virtual fish fitness-maximising through adaptive behaviours. Distribution and growth patterns emerged from fish movement and feeding behaviour. Virtual fish adapted by i) moving between patches and ii) varying the time allocated to feeding and resting behaviour to maximise growth. Fish movement in a time step was limited to patches within a distance of one patch distance upstream or downstream. Virtual fish sensed patch variables within the movement distance. They interacted through competition for feeding space and the presence of other fish changed the patch predator:prey ratio that fish used in MPR and CR>PR movement rules. Sources of stochasticity were i) the starting body mass of virtual fish (drawn from a normal distribution with the mean ± SD calculated from the field study); ii) fish of the same age were processed in a random order and iii) a fish chose a patch at random if available patches returned the same measure of fitness. There were no collectives during a simulation as a virtual fish represented a single individual. Observation was through the creation of global, patch and virtual fish files at time steps that matched the times fish and PIT-tag surveys occurred during the field study.
Details
Initialisation
The virtual environment was called in as external files. Each stretch started with the number of YOY Atlantic salmon, YOY brown trout and 1+ brown trout we collected from the first fish survey. Simulated fish had their initial starting mass drawn from a normal distribution with mean ± SD taken from stretch, age and species-specific field study recorded fish mass (see Supplementary Table S2).
Input data
In the external files, each state variable had a value for each time step. An external file also defined constant virtual fish state variables and the submodels that determined dynamic state variables.
Environment, fish bioenergetic and behavioural submodels
For reasons of space limitation, only factual descriptions of the processes essential for understanding movement behaviour and consumption are provided here. See Supplementary Methods for a complete description of the functions and justification.
Patch depth and water velocity
Patch depth and velocity were calculated for each time step using a quasi 1-D hydrological model that incorporated river discharge and accounted for spatial and temporal variability in the growth of Ranunculus spp., the dominant aquatic macrophyte.
Resource density (10 categories) and energetic content
Mean dry biomass and energy density of collected invertebrates during the field study for each prey category was calculated using length-mass59,60,61 and mass-energy relationships62. Energy density of model invertebrates was set at the calculated mean of 22.13 kJ.g dry weight−1.
Feeding and territory
Virtual fish were only able to feed in flowing water. In order to feed, a fish had to be able to occupy a patch of flowing water equal to its territory size (TS). The TS of virtual fish was size and age dependent. For each patch, its ‘Available Feeding Area’ (AFA) was the area remaining after the total area of fish territories of feeding fish currently occupying it. Virtual fish were only able to feed in patches where the patch AFA exceeded their territory size, i.e. they were able to establish a feeding space. However, virtual fish were still allowed to occupy patches where AFA < TS but could only rest.
Gross Energy Intake (GEI) by an individual fish
Virtual fish were modelled as diurnal obligate, drift feeders. Virtual fish held a stationary position within the current by swimming at a speed equal to patch water velocity, and consumed drifting invertebrates passing through a ‘capture window’28,63. If a prey entered this window, a fish would swim towards it at its maximum swimming speed, attempt to capture it, before returning with the water current. The time taken for this was handling time (HT, s) and captures were not always successful with its probability of capture success (PCS, %) dependent on water velocity64. The capture window was square and a function of a fish’s reaction distance to a prey item, which in turn was related to its size27.
Bioenergetics was modelled as a function of energy intake and the costs of standard respiration, swimming activity and energy loss through faeces and urea. Energy consumption was maximally limited according to empirically measured consumption maximums41,65 (Cmax); if potential consumption rates allowed for energy intakes greater than this limit, fish would feed up to the limit and then spending the rest of the time step resting (or non-feeding). The energy cost of swimming was dependent on the activity of the fish with feeding swimming speeds equal to 100% of patch water velocity but dropping to 50% when it was resting27. Energy losses through faeces and urea was set at a constant 31%41,65. Growth was the net energy intake divided by the energetic content of the fish; the formulas used in the bioenergetic model were derived as gross efficiency and account for energy used to synthesise new tissue66.
Death
Virtual fish were removed from the system if their body mass fell below the conservatively set threshold of 1 g.
Predator density and predation risk
Virtual fish were modelled to be aware of predators with vulnerability being dependent on predator gape-size67; YOY salmonids were preyed on by both pike sizes, and considered the density of both small and larger predators in their movement, whilst 1+ fish only considered the density of large predator. Predation was incorporated as a predatory:prey ratio at the scale of the patch.
Stage 3 – Model simulation and validation
Simulation Experiments-Alternative habitat selection behaviours
Four different habitat selection behaviours were investigated:
-
1
Random – virtual fish moved randomly.
-
2
Maximise consumption rate (MCR) – virtual fish selected the patch that had the highest consumption rate.
-
3
Minimise predation risk (MPR) – virtual fish selected to the patch with the lowest predator:prey ratio.
-
4
Prioritise maximum consumption rate then consider predation risk (CR>PR) – Virtual fish only considered predation risk if the rate of consumption was equal or greater to Cmax and if so, selected the patch with the lowest predator:prey ratio. Otherwise, virtual fish moved to the patch with the highest consumption rate.
Model Variation
100 replicate simulations were run to measure between simulation variation of fish SGR (natural flow period, % body mass.day−1) and that five replicates allowed results to be within predicted ±0.06%, 0.05% and 0.01% for YOY Atlantic Salmon, YOY and 1+ brown trout respectively.
Model validation
Pattern-orientated validation29,40 was applied using time series data of: i) body mass, ii) specific-growth rates, distribution at the iii) stretch and iv) patch scale for YOY Atlantic salmon, YOY brown trout and 1+ brown trout. Model outputs were selected to match observations by date and location when data was collected relative to the study period. Bayesian estimation of the probability of the difference between observed and predicted growth rates were calculated using the R package BEST.R68.
Fish mass and specific growth rates
We compared observed and predicted fish body masses (g) at the time points when the last three fish surveys were performed. We also compared SGRs of real and virtual fish at the end of both the NF and MLF periods as:  where BM = body mass (g), t = time (days) at the start (s) and end (e) between recaptures. Observed SGRs was calculated from recaptured, PIT tagged fish.
where BM = body mass (g), t = time (days) at the start (s) and end (e) between recaptures. Observed SGRs was calculated from recaptured, PIT tagged fish.
Stretch and patch distribution
We compared distribution at the scale of the stretch and the patch. For stretches, we calculated the percentage of the ‘total’ population located within each stretch, but removed stretches for the ‘total’ population where the probability of capture during the electric fishing surveys <0.256.
We defined the observed patch distribution of real fish from the number of tagged fish recorded per patch from the PIT-tag tracking surveys. The distribution of virtual fish was the patch location of virtual fish that were also ‘tagged’ at time steps that corresponded to when the PIT tag tracking surveys were performed during the field study.
Sensitivity analysis
To test model sensitivity, parameters sourced from literature were adjusted (±5%) and predicted SGR were compared with the unadjusted model26.
Additional Information
How to cite this article: Phang, S. C. et al. FishMORPH - An agent-based model to predict salmonid growth and distribution responses under natural and low flows. Sci. Rep. 6, 29414; doi: 10.1038/srep29414 (2016).
References
Vörösmarty, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010).
Dudgeon, D. et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (2006).
World Water Assessment Programme. The United Nations World Water Development Report 3: Water in a Changing World. (2009). Available at: http://webworld.unesco.org/water/wwap/wwdr/wwdr3/pdf/WWDR3_Water_in_a_Changing_World.pdf. (Date of access: 06/11/2011).
Harrod, J. J. The Distribution of Invertebrates on Submerged Aquatic Plants in a Chalk Stream. J. Anim. Ecol. 33, 335–348 (1964).
Neale, M. W., Dunbar, M. J., Jones, J. & Ibbotson, A. T. A comparison of the relative contributions of temporal and spatial variation in the density of drifting invertebrates in a Dorset (UK) chalk stream. Freshw. Biol. 53, 1513–1523 (2008).
Berrie, A. D. The chalk-stream environment. Hydrobiologia 248, 3–9 (1992).
Carpenter, S. R., Chisholm, S. W., Krebs, C. J., Schindler, D. W. & Wright, R. F. Ecosystem Experiments. Science (80-.). 269, 324–327 (1995).
Murphy, J. M. et al. (2009). Available at: http://ukclimateprojections.metoffice.gov.uk/media.jsp?mediaid=87893. (Date of access: 06/25/2011).
Maddock, I. The importance of physical habitat assessment for evaluating river health. Freshw. Biol. 41, 373–391 (1999).
Guisan, A. & Thuiller, W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (2005).
Caro, T. Behavior and conservation: a bridge too far? Trends Ecol. Evol. 22, 394–400 (2007).
Herricks, E. E. & Bergner, E. R. Prediction of climate change effects of fish communities in the Mackinaw River watershed, Illinois, USA. Water Sci. Technol. 48, 199–207 (2003).
Lawrence, D. J. et al. The interactive effects of climate change, riparian management, and a nonnative predator on stream-rearing salmon. Ecol. Appl. 24, 895–912 (2013).
Xenopoulos, M. A. et al. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Glob. Chang. Biol. 11, 1557–1564 (2005).
Hunt, R. L. Overwinter Survival of Wild Fingerling Brook Trout in Lawrence Creek, Wisconsin. J. Fish. Res. Board Canada 26, 1473–1483 (1969).
Francova, K. & Ondrackova, M. Overwinter body condition, mortality and parasite infection in two size classes of 0+ year juvenile European bitterling Rhodeus amarus. J. Fish Biol. 82, 555–568 (2013).
Heggenes, J., Bagliniere, J. L. & Cunjak, R. A. Spatial niche variability for young Atlantic salmon (Salmo salar) and brown trout (S-trutta) in heterogeneous streams. Ecol. Freshw. Fish 8, 1–21 (1999).
Heggenes, J., Saltveit, J. & Lingaas, O. Predicting fish habitat use to changes in water flow: Modelling critical minimum flows for Atlantic salmon, Salmo salar, and brown trout, S-trutta. Regul. Rivers-Research Manag. 12, 331–344 (1996).
Keeley, E. R. An experimental analysis of territory size in juvenile steelhead trout. Anim. Behav. 59, 477–490 (2000).
Johnsson, J. I., Nöbbelin, F. & Bohlin, T. Territorial competition among wild brown trout fry: effects of ownership and body size. J. Fish Biol. 54, 469–472 (1999).
Grant, J. W. A. & Kramer, D. L. Territory Size as a Predictor of the Upper Limit to Population Density of Juvenile Salmonids in Streams. Can. J. Fish. Aquat. Sci. 47, 1724–1737 (1990).
Sutherland, W. J. & Freckleton, R. P. Making predictive ecology more relevant to policy makers and practitioners. Philos. Trans. R. Soc. B Biol. Sci. 367, 322–330 (2012).
Railsback, S. F. & Grimm, V. Agent-Based And Individual-Based Modelling. (Princeton University Press, USA, 2012).
Stillman, R. A. MORPH–An individual-based model to predict the effect of environmental change on foraging animal populations. Ecol. Modell. 216, 265–276 (2008).
Stillman, R. A. et al. Assessing waterbird conservation objectives: An example for the Burry Inlet, UK. Biol. Conserv. 143, 2617–2630 (2010).
Stillman, R. A., West, A. D., Caldow, R. W. G. & Durell, S. E. A. L. V. D. Predicting the effect of disturbance on coastal birds. Ibis (Lond. 1859). 149, 73–81 (2007).
Railsback, S. F., Harvey, B. C., Jackson, S. K. & Lamberson, R. H. InSTREAM: the individual-based streamtrout research and environmental assessment model. Gen. Tech. Rep. PSW-GTR-218. (2009).
Railsback, S. F. & Harvey, B. C. Analysis of habitat-selection rules using an individual-based model. Ecology 83, 1817–1830 (2002).
Grimm, V. & Railsback, S. F. Pattern-oriented modelling: a ‘multi-scope’ for predictive systems ecology. Philos. Trans. R. Soc. B Biol. Sci. 367, 298–310 (2012).
Piñeiro, G., Perelman, S., Guerschman, J. P. & Paruelo, J. M. How to evaluate models: Observed vs. predicted or predicted vs. observed? Ecol. Modell. 216, 316–322 (2008).
Cucherousset, J. et al. Determining the effects of species, environmental conditions and tracking method on the detection efficiency of portable PIT telemetry. J. Fish Biol. 76, 1039–1045 (2010).
Murphy, M. H., Connerton, M. J. & Stewart, D. J. Evaluation of winter severity on growth of young-of-the-year Atlantic salmon. Trans. Am. Fish. Soc. 135, 420–430 (2006).
Smith, R. W. & Griffith, J. S. Survival of Rainbow Trout during Their First Winter in the Henrys Fork of the Snake River, Idaho. Trans. Am. Fish. Soc. 123, 747–756 (1994).
Horth, N. A. & Dodson, J. J. Influence of individual body size and variable thresholds on the incidence of sneaker maler reproductive tactiv in Atlantic salmon. Evolution (N. Y). 58, 136–144 (2004).
Quinn, T. P. & Foote, C. The effects of body size and sexual dimorphism on the reproductive behaviour of sockeye salmon, Oncorhynchus nerka. Anim. Behav. 48, 751–761 (1994).
Mann, R. H. K. The Annual Food Consumption and Prey Preferences of Pike (Esox lucius) in the River Frome, Dorset. J. Anim. Ecol. 51, 81–95 (1982).
Savino, J. F. & Stein, R. A. Behavior of fish predators and their prey: habitat choice between open water and dense vegetation. Environ. Biol. Fishes 24, 287–293 (1989).
Mann, R. H. K. A pike management strategy for a trout fishery. J. Fish Biol. 27, 227–234 (1985).
Stillman, R. A., Railsback, S. F., Giske, J., Berger, U. & Grimm, V. Making Predictions in a Changing World: The Benefits of Individual-Based Ecology. Biosci. 65, 140–150 (2015).
Grimm, V. & Railsback, S. F. Individual-based Modelling and Ecology. (Princeton University Press, 2005).
Elliott, J. M. The Growth Rate of Brown Trout (Salmo trutta L.) Fed on Maximum Rations. J. Anim. Ecol. 44, 805–821 (1975).
Pinder, A. C., Riley, W. D., Ibbotson, A. T. & Beaumont, W. R. C. Evidence for an autumn downstream migration and the subsequent estuarine residence of 0+ year juvenile Atlantic salmon Salmo salar L., in England. J. Fish Biol. 71, 260–264 (2007).
Folmar, L. C. & Dickhoff, W. W. The parr—Smolt transformation (smoltification) and seawater adaptation in salmonids. Aquaculture 21, 1–37 (1980).
Björnsson, B. T., Stefansson, S. O. & McCormick, S. D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 170, 290–298 (2011).
McCormick, S. D., Hansen, L. P., Quinn, T. P. & Saunders, R. L. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 55, 77–92 (1998).
Hoogenboom, M. O., Armstrong, J. D., Groothuis, T. G. G. & Metcalfe, N. B. The growth benefits of aggressive behavior vary with individual metabolism and resource predictability. Behav. Ecol. 24, 253–261 (2012).
Grimm, V. et al. Pattern-Oriented Modeling of Agent-Based Complex Systems: Lessons from Ecology. Science (80-.). 310, 987–991 (2005).
Titus, R. G. Territorial behavior and its role in population regulation of young brown trout (Salmo trutta): new perspectives. Ann. Zool. Fennici 27, 119–130 (1990).
Wright, J. F. & Symes, K. L. A nine-year study of the macroinvertebrate fauna of a chalk stream. Hydrol. Process. 13, 371–385 (1999).
Imre, I., Grant, J. W. A. & Keeley, E. R. The effect of food abundance on territory size and population density of juvenile steelhead trout (Oncorhynchus mykiss). Oecologia 138, 371–378 (2004).
Railsback, S. F. Getting ‘results’: the pattern-orientated approach to analyzing natural systems withi individual-based models. Nat. Resour. Model. 14, 465–475 (2001).
van der Vaart, E., Beaumont, M. A., Johnston, A. S. A. & Sibly, R. M. Calibration and evaluation of individual-based models using Approximate Bayesian Computation. Ecol. Modell. 312, 182–190 (2015).
Zurell, D. et al. The virtual ecologist approach: simulating data and observers. Oikos 119, 622–635 (2010).
Faulkner, H. & Copp, G. H. A model for accurate drift estimation in streams. Freshw. Biol. 46, 723–733 (2001).
Cucherousset, J. et al. Fitness consequences of individual specialisation in resource use and trophic morphology in European eels. Oecologia 167, 75–84 (2011).
Seber, G. A. F. & Cren, E. D. Le. Estimating Population Parameters from Catches Large Relative to the Population. J. Anim. Ecol. 36, 631–643 (1967).
Grimm, V. et al. The ODD protocol: a review and first update. Ecol. Modell. 221, 2760–2768 (2010).
Grimm, V. et al. A standard protocol for describing individual-based and agent-based models. Ecol. Modell. 198, 115–126 (2006).
Benke, A. C., Huryn, A. D., Smock, L. A. & Wallace, J. B. Length-Mass Relationships for Freshwater Macroinvertebrates in North America with Particular Reference to the Southeastern United States. J. North Am. Benthol. Soc. 18, 308–343 (1999).
Ganihar, S. Biomass estimates of terrestrial arthropods based on body length. J. Biosci. 22, 219–224 (1997).
Sabo, J. L., Bastow, J. L. & Power, M. E. Length-Mass Relationships for Adult Aquatic and Terrestrial Invertebrates in a California Watershed. J. North Am. Benthol. Soc. 21, 336–343 (2002).
Cummins, K. W. & Wuycheck, J. C. Caloric Equivalents For Investigations In Ecological Energetics. (International Assocation of Theoretical and Applied Limnology, Stuttgart, Germany, 1971).
Hayes, J. W., Stark, J. D. & Shearer, K. A. Development and Test of a Whole-Lifetime Foraging and Bioenergetics Growth Model for Drift-Feeding Brown Trout. Trans. Am. Fish. Soc. 129, 315–332 (2000).
Piccolo, J., Hughes, N. & Bryant, M. Water velocity influences prey detection and capture by drift-feeding juvenile coho salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss irideus). Can. J. Fish. Aquat. Sci. 65, 266–275 (2008).
Elliott, J. M. The Growth Rate of Brown Trout (Salmo trutta L.) Fed on Reduced Rations. J. Anim. Ecol. 44, 823–842 (1975).
Elliott, J. M. The Energetics of Feeding, Metabolism and Growth of Brown Trout (Salmo trutta L.) in Relation to Body Weight, Water Temperature and Ration Size. J. Anim. Ecol. 45, 923–948 (1976).
Nilsson, P. A. & Brönmark, C. Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88, 539–546 (2000).
Kruschke, J. K. Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General 142, 573–603 (2013).
Acknowledgements
We are grateful to all our colleagues that helped with the collection of data in the field study. We thank S. Gregory for improving the manuscript. C.J. was supported by an “ERG Marie Curie” grant (PERG08-GA-2010- 276969).
Author information
Authors and Affiliations
Contributions
P.S.C. wrote the manuscript text and prepared the figures. P.S.C., C.J., G.R., B.J.R., R.D. and B.W.R.C. helped with data collection. P.S.C., B.J.R., C.J., G.R. and S.R.A. constructed and tested the model. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Phang, S., Stillman, R., Cucherousset, J. et al. FishMORPH - An agent-based model to predict salmonid growth and distribution responses under natural and low flows. Sci Rep 6, 29414 (2016). https://doi.org/10.1038/srep29414
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29414
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.