Abstract
Candida albicans and Fusobacterium nucleatum are well-studied oral commensal microbes with pathogenic potential that are involved in various oral polymicrobial infectious diseases. Recently, we demonstrated that F. nucleatum ATCC 23726 coaggregates with C. albicans SN152, a process mainly mediated by fusobacterial membrane protein RadD and Candida cell wall protein Flo9. The aim of this study was to investigate the potential biological impact of this inter-kingdom interaction. We found that F. nucleatum ATCC 23726 inhibits growth and hyphal morphogenesis of C. albicans SN152 in a contact-dependent manner. Further analysis revealed that the inhibition of Candida hyphal morphogenesis is mediated via RadD and Flo9 protein pair. Using a murine macrophage cell line, we showed that the F. nucleatum-induced inhibition of Candida hyphal morphogenesis promotes C. albicans survival and negatively impacts the macrophage-killing capability of C. albicans. Furthermore, the yeast form of C. albicans repressed F. nucleatum-induced MCP-1 and TNFα production in macrophages. Our study suggests that the interaction between C. albicans and F. nucleatum leads to a mutual attenuation of virulence, which may function to promote a long-term commensal lifestyle within the oral cavity. This finding has significant implications for our understanding of inter-kingdom interaction and may impact clinical treatment strategies.
Similar content being viewed by others
Introduction
The human oral cavity is arguably one of the most complex microbial ecosystems identified to date1,2,3. While the majority of oral microbial residents are bacteria (>600 phylotypes), studies have also revealed the presence of diverse fungal species, with Candida albicans as the most prevalent4,5. In healthy hosts, C. albicans often exists as a harmless microorganism within the oral microbial community6. However, under conditions of immune dysfunction or local predisposition factors such as poor oral hygiene, C. albicans can become a clinically significant opportunistic pathogen and cause recurrent mucosal infection or life-threatening disseminated infections7,8,9.
As an important non-bacterial constituent of the human-associated microbiota, C. albicans displays diverse inter-kingdom interactions that range from synergistic to antagonistic6,10. For example, interactions between C. albicans and Staphylococcus aureus lead to enhanced pathogenic behavior and disease severity through physical as well as metabolic interactions11,12. Synergistic relationships have also been documented between C. albicans and oral streptococci, resulting in enhanced dual-species biofilm formation and increased antibiotic resistance13,14. Conversely, Lactobacillus spp. are able to antagonize C. albicans, possibly through hydrogen peroxide production and other yet-to-be determined mechanisms15. These Candida-bacterial interactions have been implicated in contributing to polymicrobial disease processes and impacting disease outcomes16.
Fusobacterium nucleatum is a Gram-negative anaerobe, important in plaque and biofilm formation in the oral cavity. Due to its ability to form physical interactions with Gram-positive and Gram-negative species, F. nucleatum is a well-known “bridging” organism essential for the ordered succession of colonization events in oral polymicrobial communities17,18,19.
Co-localization and physical interaction between oral isolates of C. albicans and F. nucleatum have been well-documented20,21. In a recent study, we demonstrated the physical co-adherence between F. nucleatum ATCC 23726 and C. albicans SN152. By screening a C. albicans SN152 mutant library and a panel of F. nucleatum 23726 outer membrane protein mutants, we further identified C. albicans cell wall protein Flo9 and F. nucleatum membrane protein RadD as the main components mediating the interaction22. In the current study, we sought to elucidate the biological impact of this inter-kingdom interaction by investigating its consequences on growth, morphology and virulence of C. albicans and F. nucleatum. We found that F. nucleatum prevents the transition of C. albicans from yeast to hyphae in a contact-dependent manner, which leads to an attenuation of macrophage response. In addition, we found evidence for a mutual tempering of virulence properties between C. albicans and F. nucleatum that may allow for a mutually beneficial co-existence with the host.
Results
F. nucleatum ATCC 23726 inhibits hyphal morphogenesis and growth of Candida albicans SN152 yeast cells
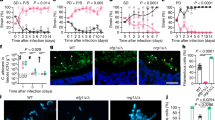
In a recent study, we demonstrated physical co-adherence between F. nucleatum ATCC 23726 and C. albicans SN15222. To further investigate the biological relevance of the interactions between these two clinically relevant microbes, C. albicans yeast cells were grown alone or co-cultured with F. nucleatum in fetal bovine serum (FBS)-containing, hyphae-inducing yeast extract peptone-dextrose (YPD) medium. In monoculture, C. albicans yeast cells developed hyphae after a 4-hour incubation (Fig. 1-A1); while co-cultivation with F. nucleatum resulted in only yeast and pseudohyphae formation in C. albicans (Fig. 1-A2). In addition, when grown in monoculture, C. albicans colony-forming units (CFU) increased ~10-fold after 4 hours, while no apparent increase in CFU was observed for C. albicans during its co-cultivation with F. nucleatum (Fig. 1-B1). No significant growth was observed for F. nucleatum when it was cultivated as mono-culture or co-cultivated with C. albicans in the same FBS-containing YPD medium (Fig. 1-B2). Interestingly, when C. albicans was co-incubated with Streptococcus mutans, another oral commensal bacterium, for the same amount of time, a 10-fold increase in C. albicans growth was observed and we did not observe any inhibition in hyphal morphogenesis (Fig. S1).
Effects of F. nucleatum (Fn) on the growth and hyphal morphogenesis of C. albicans (Ca).
After 4-hour incubation under Ca hyphae-inducing conditions described in the Materials and Methods, samples from monoculture of Ca yeast cells (A1), or Ca yeast/Fn (A2) and Ca hyphae/Fn (A3) co-cultures were taken and visualized under the microscope. At least 10 images were taken for each sample and representative images are shown. The viability of Ca (B1) and Fn (B2) was monitored before and after their 4-hour incubation as mono- and duo-species. The viability of Ca was also determined when pre-developed Ca hyphae were co-cultured with Fn (B3). The lower-right inset in A1 shows Ca yeast cells before cultivation. Error bars = SD. A star indicates P < 0.05. The scale bar is 10 μm.
We also investigated the impact of F. nucleatum on the growth of the preformed hyphal C. albicans. The results show that while C. albicans hyphae achieved a 10-fold increase in cell number after 4 hours cultivation as monoculture; no significant growth was observed when co-cultivated with F. nucleatum, suggesting that F. nucleatum is able to inhibit the growth of hyphal C. albicans as well (Fig. 1-B3). Furthermore, no obvious change in the morphology of C. albicans’ hyphae was observed during co-cultivation with F. nucleatum (Fig. 1-A3).
F. nucleatum ATCC 23726 inhibits hyphal morphogenesis of C. albicans SN152 in a contact-dependent manner
To determine if the observed F. nucleatum-induced hyphal morphogenesis inhibition requires direct cell–cell contact, we separated C. albicans yeast cells and F. nucleatum in a two-chamber vessel separated by a 0.4-μm membrane. Two-chamber assays have been used for distinguishing between cell-cell contact-dependent and diffusible signal-dependent microbial interactions in previous studies23,24. Organisms in each chamber are physically separated by the membrane, but share the same growth medium. As illustrated in Fig. 2A, F. nucleatum was incubated in the lower chamber and C. albicans was cultured in the upper chamber. Interestingly, after 4-hour incubation, we did not observe inhibition of hyphal morphogenesis when C. albicans yeast cells and F. nucleatum were physically separated by the membrane (Fig. 2A).
Contact-dependent hyphal morphogenesis inhibition of C. albicans.
C. albicans (Ca) wt (A–C) or Ca flo9 mutant (D) yeast cells were inoculated into the upper chamber, while mono-culture of F. nucleatum (Fn) wt (A), co-cultures of Ca wt yeast cells and Fn wt (B); Ca wt yeast cells and Fn radD mutants (C); or Ca flo9 yeast cells and Fn wt (D) were inoculated into the lower chamber. Microbes in the two chambers are physically separated by a 0.4-μm pore-size membrane, but share the same culture medium. Chambers were incubated as described in Materials and Methods. Samples in both chambers were taken 4 hours after cultivation and visualized under the microscope. All assays were performed in duplicate and repeated three times on different days. At least 10 images were taken for each sample and representative images are presented. The scale bar is 10 μm.
However, when C. albicans yeast cells were added to the F. nucleatum culture in the lower chamber, hyphal morphogenesis was inhibited, while the C. albicans monoculture in the upper chamber was not affected (Fig. 2B). Furthermore, the spent medium of 4-hour old F. nucleatum monoculture or C. albicans/F. nucleatum co-culture did not show a hyphal morphogenesis inhibition effect (data not shown). These results suggest that the observed hyphal morphogenesis inhibition requires direct cell contact between C. albicans and F. nucleatum.
Cell wall protein Flo9 of C. albicans and membrane protein RadD of F. nucleatum mediate the inhibition of hyphal morphogenesis
Our recent study indicated the presence of multiple receptor/ligand pairs involved in the physical co-adherence between C. albicans SN152 and F. nucleatum 23726; specifically revealing cell wall protein Flo9 (C. albicans) and membrane protein RadD (F. nucleatum) as the main cellular components mediating the interaction22. In an effort to investigate if the same membrane component pair is involved in the observed contact-dependent hyphal morphogenesis inhibition, C. albicans flo9 and F. nucleatum radD mutants were used to carry out a similar two-chamber assay. Results showed that unlike F. nucleatum wild type, the radD mutant failed to inhibit hyphal formation in C. albicans wild type during co-cultivation in the same chamber (Fig. 2C), while C. albicans flo9 mutant portrayed normal hyphal morphogenesis even when co-cultivated with wild type F. nucleatum in the lower chamber (Fig. 2D). These data indicated that Flo9 and RadD are indeed involved in the contact-dependent hyphal morphogenesis inhibition.
F. nucleatum inhibits the growth of yeast C. albicans in a contact-dependent but Flo9/RadD-independent manner
To investigate if the same membrane components are required for the observed F. nucleatum-induced growth inhibition of C. albicans, we characterized the growth of C. albicans yeast cells using a two-chamber assay. When co-cultured in the same chamber with wild type F. nucleatum, there was not a significant increase in C. albicans CFU after 4 hours. However, a 10-fold increase in CFU was observed when C. albicans was cultured in a separate chamber while sharing the growth medium with F. nucleatum (Fig. 3(A1, A2), suggesting the growth inhibition requires direct cell-contact between C. albicans and F. nucleatum and is unlikely mediated via diffusible signals. This was further supported by the observation that the spent medium of 4-hour old F. nucleatum monoculture or C. albicans/F. nucleatum co-culture did not display a growth inhibitory effect against C. albicans (data not shown). Furthermore, growth inhibition of C. albicans was also observed when C. albicans wild type or the flo9 mutant was co-cultured in the same chamber with F. nucleatum wild type or radD mutant (Fig. 3B,C). This indicates that the F. nucleatum-induced contact-dependent growth inhibition of C. albicans is not mediated by the Flo9 or RadD protein, but rather by additional as yet uncharacterized receptor/ligand pairs that are also involved in the physical co-adherence between C. albicans and F. nucleatum22.
Contact-dependent growth inhibition of C. albicans.
Yeast cells of C. albicans (Ca) wt or flo9 mutant were inoculated into the upper chamber, while co-cultures of Ca wt and F. nucleatum (Fn) wt (A1); Ca wt and Fn radD (B1); or Ca flo9 and Fn wt (C1) were inoculated into the lower chamber. Chambers were incubated at 37 °C for 4 hours. Samples in both chambers were taken before or 4 hours after incubation and Ca viability was monitored as described in Materials and Methods. All assays were performed in duplicate and repeated three times on different days. Error bars = SD. A star indicates P < 0.05.
F. nucleatum-induced yeast and pseudohyphal forms of C. albicans display reduced sensitivity to RAW macrophage killing
Macrophages are part of the innate immunity and first responders to disease sites. Therefore, they readily interact with both F. nucleatum and C. albicans. We used the murine-derived macrophage RAW 264.7 cell line as an in vitro model to investigate the impact of F. nucleatum-induced hyphal morphogenesis inhibition on host-microbe interactions. First, the yeast or hyphal C. albicans alone was co-incubated with RAW cells at an MOI (multiplicity of infection) of 1:1 for 90 minutes. Viability of C. albicans was monitored as described in Materials and Methods. Interestingly, after incubating with macrophages, C. albicans hyphae suffered 4-5-fold loss of cell viability, while no significant reduction in viability was observed for similarly treated C. albicans yeast cells (Fig. 4), suggesting that C. albicans hyphae are more sensitive to macrophage killing than yeast cells under the conditions we tested. Next, we challenged RAW cells with F. nucleatum and either C. albicans yeast cells or pre-developed hyphae for 90 min. During the experimental period, C. albicans maintained yeast or pseudohyphal form due to the presence of F. nucleatum. The results show that, when co-cultured with RAW cells and F. nucleatum, pre-developed hyphal C. albicans suffered about 75% reduction in CFU after 90 minutes incubation, while we did not see a significant difference in CFU of yeast and pseudohyphal C. albicans after the same incubation period (Fig. 4). These data suggest that F. nucleatum prevents yeast-to-hyphae transition in C. albicans, which allows C. albicans to survive better in the presence of macrophages (Fig. 4). In addition, we also showed that F. nucleatum viability was not significantly affected during the 90-minute incubation with RAW cells and C. albicans (Fig. S2).
Killing of C. albicans (Ca) cells by RAW macrophages.
The yeast or hyphal Ca was incubated in the presence or absence of RAW cells with or without F. nucleatum(Fn) for 90 minutes. C. albicans cell viability was determined as described in Materials and Methods and expressed as the percentage of viable cells 90 minutes after incubation compared to the starting cell number. Three independent experiments were carried out under each condition. Error bars = SD. A star indicates P < 0.05.
C. albicans represses F. nucleatum-induced Monocyte Chemotactic Protein 1 (MCP-1) and Tumor Necrosis Factor-α (TNFα) production in macrophages
To further investigate the impact of the interaction between C. albicans and F. nucleatum on host response, we measured the induction of MCP-1 and TNFα in RAW macrophage cells using a plate-based ELISA assay (see Materials and Methods). These two cytokines were chosen because of their importance during the initial macrophage response. In addition, our initial screen showed that these were produced at high levels by RAW cells in the presence of F. nucleatum. RAW cells were co-incubated with C. albicans or F. nucleatum alone or in combination. The results show that F. nucleatum induced a 5-fold increase in the production of MCP-1, while yeast or hyphal C. albicans alone resulted in a moderate 2-3-fold increase (Fig. 5A). We also observed a similar trend in TNFα production; incubation with F. nucleatum led to drastically increased TNFα production whereas both yeast and hyphal C. albicans alone had only a moderate effect (Fig. 5B). Interestingly, the production of MCP-1 and TNFα induced by F. nucleatum was significantly reduced when macrophages were co-incubated with F. nucleatum and yeast C. albicans. In addition, we observed that compared to the yeast form, the hyphal C. albicans displayed less inhibition on F. nucleatum-induced MCP-1 and TNFα production in RAW cells and it was not statistically significant (Fig. 5).
Differential induction of Monocyte Chemotactic Protein 1 (MCP-1) and Tumor Necrosis Factor (TNFα) in RAW macrophages.
RAW cells were incubated with different combinations of C. albicans (Ca) and F. nucleatum (Fn) for 4 hours. Release of MCP-1 (A) and TNFα (B) proteins in the supernatant were quantified by an ELISA-based standard assay (see Materials and Methods). Three independent experiments were carried out under each condition. Error bars = Standard Error of the Mean. A star indicates significant differences (P < 0.05) between values of the two groups linked by lines above them.
F. nucleatum negatively impacts the macrophage-killing ability of C. albicans
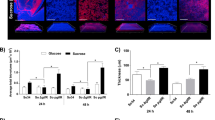
Both C. albicans and F. nucleatum have been shown to induce host immune cell death25,26. To investigate the impact of interspecies interaction between C. albicans and F. nucleatum on macrophage-killing ability, RAW macrophage cells were challenged with mono- or co-culture of C. albicans and F. nucleatum. The viability of the macrophages was monitored using propidium iodide (PI), a stain that fluorescently labels the DNA of cells with compromised membranes and thus is a marker for cell death. PI signal was visualized with fluorescent microscopy (see Materials and Methods). As shown in Fig. 6, when RAW macrophages were challenged with F. nucleatum alone at an MOI of 1:1000, there was a higher PI signal at all time points compared to RAW cells alone (Fig. 6A,B). When RAW cells were challenged with C. albicans monoculture (either hyphae or yeast cells) at an MOI of 1:10, the amount of PI staining was similar to RAW cells challenged with F. nucleatum at the early time points (2 and 4 hours); while more signal was observed at the later time point (6 hours) (Fig. 6C,D). Notably, exposure to C. albicans (hyphae or yeast cells)/F. nucleatum co-culture resulted in drastically less PI signal compared to samples challenged with C. albicans alone, with a level of PI staining similar to that observed when RAW cells were challenged with F. nucleatum alone (Fig. 6E,F). Under the specific conditions for this experiment, C. albicans developed hyphae after 6 hours even in the presence of F. nucleatum; therefore, we limited the data collection to 6 hours. Our data suggest that F. nucleatum is able to reduce the killing ability of both yeast and hyphal C. albicans against RAW cells.
Propidium iodide (PI) staining of Macrophages after challenge with C. albicans (Ca) and/or F. nucleatum (Fn).
Overnight seeded macrophages were challenged with different combinations of Ca and/or Fn for 2, 4 and 6 hours. The incubation media contains PI, a fluorescent stain which only incorporates into cells that have a compromised membrane. Experiment was performed in triplicate and representative images are shown. The scale bars are 100 μm.
Discussion
Increasing lines of evidence have revealed extensive interactions between C. albicans and various bacterial species within host-associated multi-species microbiota10,27. These inter-kingdom interactions may be crucial to the persistence of C. albicans within the host as part of the commensal flora and potentially contribute to the progression of polymicrobial infections under certain conditions16. In a recent study, we demonstrated the direct physical interaction between C. albicans and F. nucleatum, an oral commensal microbe with pathogenic potential and identified the main membrane components mediating the co-adherence22. In the current study, we further investigated the biological consequences and relevance of these interactions.
Our data clearly reveal that F. nucleatum ATCC 23726 inhibits hyphal morphogenesis of C. albicans SN152 in a contact-dependent manner (Figs 1 and 2). Unlike its yeast form, which is more often isolated from healthy subjects, hyphal C. albicans is usually more dominant in diseased patients and considered to be more virulent16,27. Thus, the bacteria-induced C. albicans hyphal morphogenesis inhibition may serve to attenuate the virulence of the fungus and potentially reflects an evolutionary mechanism meant to keep C. albicans in balance with the host28.
Hyphal morphogenesis inhibition in C. albicans has been shown to occur during interactions with a variety of host-associated bacterial species, including Burkholderia cenocepacia29, Xanthomonas campestris30, Salmonella enterica subsp. enterica serovar Typhimurium31, Pseudomonas spp.32 and Enterococcus faecalis33. Intriguingly, all examples of bacteria-induced hyphal morphogenesis inhibition reported to date involve diffusible signal factors. For example, P. aeruginosa-generated 3-oxo-C12-homoserine lactone34, as well as decanoic acids produced by B. cenocepacia29 and X. campestris30, mimic the C. albicans quorum sensing molecule farnesol, leading to repression of hyphal morphogenesis. Similarly, unidentified heat-stable secretory molecules produced by S. Typhimurium31 and E. faecalis33 have also been implicated in mediating hyphal morphogenesis inhibition. In many cases, these diffusible inhibitory signals are produced even when C. albicans is not present33, indicating a non-specific response. On the contrary, our results show that the F. nucleatum-induced hyphal morphogenesis inhibition in C. albicans is mediated by the membrane protein pair RadD/Flo9 (Fig. 2), which has been shown to facilitate the physical adherence between F. nucleatum and C. albicans22, suggesting a more specific cross-kingdom interaction.
In addition, we also revealed F. nucleatum-induced contact-dependent growth inhibition against C. albicans. The observed growth inhibition is not likely due to nutrition depletion, since in the two-chamber assay, growth inhibition in C. albicans was detected only when it was co-cultivated with F. nucleatum in the same chamber, while no inhibition was detected when it was physically separated from F. nucleatum by the membrane while still sharing the same growth medium (Fig. 3). Interestingly, RadD and Flo9 are not required for the F. nucleatum-induced contact-dependent growth inhibition against C. albicans (Fig. 3), implying that even though both events are contact-dependent, inhibition of C. albicans growth and hyphal morphogenesis are likely mediated by different membrane components. Our previous study showed that while RadD/Flo9 is the main protein pair mediating the observed co-aggregation between C. albicans and F. nucleatum, other uncharacterized receptor/ligand pairs also contribute to the co-adherence, although to a lesser extent22. These yet-to-be identified components could play a role in the observed contact-dependent growth inhibition.
It is worth mentioning that P. aeruginosa and S. Typhimurium can both inhibit C. albicans growth by binding to hyphae and inducing killing via secreted virulence factors (hemolytic phospholipase C)32,35 or through the type III secretion system36, respectively. However, in the case of C. albicans and F. nucleatum, our results show that the binding of F. nucleatum to hyphal C. albicans does not induce obvious killing (Fig. 1B3). The observed growth inhibition incurred by F. nucleatum against C. albicans hyphae and yeast did not result in decreased cell viability, but rather prevented C. albicans from growth. The detailed mechanisms underlying F. nucleatum-induced contact-dependent hyphal morphogenesis and growth inhibition in C. albicans remain to be determined.
As oral commensal microbes, C. albicans and F. nucleatum experience constant interactions with host cells. Macrophages are particularly critical to the host’s ability to counteract C. albicans infection. Macrophages limit C. albicans burden during early infection and more importantly, recruit and activate other immune cells37. Using the RAW murine macrophage cell line, we showed that hyphal C. albicans is more sensitive to macrophage killing in vitro compared to the yeast form (Fig. 4). Due to a number of important physiological, structural and biochemical differences, C. albicans yeast and hyphal forms often induce a differential host immune response28,37,38,39,40. The observed higher candidacidal activity of RAW cells against the hyphal form may be the result of contact-mediated killing of hyphae41 as well as higher sensitivity of hyphae to nitrogen-containing compounds released by macrophages42. Our results suggest that interaction with F. nucleatum inhibits yeast-to-hyphae transition in C. albicans, thus retaining C. albicans in a more macrophage-resistant morphology. Furthermore, the presence of F. nucleatum not only promoted the resistance of C. albicans to macrophage killing, but also decreased the ability of C. albicans to kill macrophages through a yet-to-be determined mechanism (Fig. 6). These findings, together with the overall reduced growth of C. albicans in the presence of F. nucleatum, suggest that interaction between C. albicans and F. nucleatum might promote a less pathogenic lifestyle when existing within the host.
Interestingly, our data demonstrate that co-infection with C. albicans yeast cells reduces F. nucleatum-induced MCP-1 and TNFα production in RAW macrophages, while hyphal C. albicans does not show such a drastic negative impact (Fig. 5). MCP-1 produced by macrophages is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages, which are required for routine immunological surveillance and response to inflammation43. TNFα is one of the main cytokines produced by macrophages in response to bacterial challenge. It has a wide range of roles in immune cell proliferation, recruitment, apoptosis and activation44. As a Gram-negative bacterium, F. nucleatum alone may induce MCP-1 and TNFα production, potentially via its membrane-associated LPS45, while C. albicans seems to modulate and negatively impact the ability of F. nucleatum to induce MCP-1 and TNFα production in RAW macrophages. Although the detailed mechanism is yet to be determined, the negative impact of C. albicans on F. nucleatum’s ability to induce macrophage cytokine production is not achieved by affecting its cellular viability, since F. nucleatum viability was not affected during the 90-minute incubation with C. albicans and macrophages (Fig. S2). Most interestingly, C. albicans yeast cells are more effective in inhibiting F. nucleatum–induced MCP-1 and TNFα production compared to hyphae, suggesting that the F. nucleatum-induced hyphal morphogenesis inhibition potentially benefits both partners by attenuating host response.
C. albicans and F. nucleatum are part of the commensal oral microbial community in healthy subjects and it is to the benefit of the microbes to maintain a long-term commensal relationship with the host. A similar example of a Candida-bacterial interaction that promotes long-term commensalism with the host has been described between C. albicans and E. faecalis33. Using a Caenorhabditis elegans model to study polymicrobial infection, Cruz et al. demonstrated that co-infection with C. albicans and E. faecalis resulted in less pathology and less mortality than mono-species infection due to a mutual attenuation of virulence33. Our results are in line with this finding. By repressing the F. nucleatum-induced production of MCP-1 and TNFα in macrophages, C. albicans can limit the host innate immune response, which would otherwise result in microbial clearance. More importantly, the host defense is able to discriminate C. albicans pathogenic invasion from commensal colonization mainly through its different morphologies. While the yeast form is often regarded as a sign of normal colonization, the presence of hyphae is usually a sign of invasion which induces an extensive host response28. In this regard, the yeast/pseudohyphal C. albicans as a result of F. nucleatum-induced hyphal morphogenesis inhibition may serve to render C. albicans less pathogenic and lead to a lessened host immune response. The reduced pathogenicity is also reflected by the reduced macrophage-killing ability of C. albicans when interacting with F. nucleatum. By mutually inhibiting virulence, C. albicans and F. nucleatum trigger less of an immune response, which may allow this microbial pair to achieve long-term persistence and fitness within the host. An in vivo study testing this hypothesis is currently underway.
In summary, we have identified a specific contact-dependent inter-kingdom interaction between C. albicans and F. nucleatum that promotes a commensal rather than pathogenic lifestyle when encountering host cells. These findings, together with the recent reports of the commensalism between C. albicans and E. faecalis in the mouse gut46 suggest that through mutual inhibition of virulence, C. albicans and its interacting bacterial counterparts trade their pathogenicity for long-term fitness, thus facilitating a commensal existence within the host.
Materials and Methods
Strains and cell lines
C. albicans SN152 wild type and flo9 mutant were routinely propagated overnight at 37 °C aerobically in yeast extract peptone-dextrose (YPD) broth (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose). Hyphal C. albicans was induced by inoculating the pre-grown C. albicans yeast in fresh YPD broth supplemented with 20% fetal bovine serum (FBS, Gibco) and incubating at 37 °C aerobically for 4 hrs. For yeast or pseudo-hyphal C. albicans, colony forming units (CFU) were enumerated by plating serially-diluted C. albicans cultures on YPD agar plates and counting colonies after incubating the plates under aerobic conditions at 37 °C for 24 hrs.
F. nucleatum ATCC 23726 wild type strain as well as a radD mutant, which carries a mutation in the gene encoding outer membrane protein RadD47, were grown in Columbia broth (Becton Dickinson and Co) at 37 °C under anaerobic conditions (nitrogen 90%, carbon dioxide 5%, hydrogen 5%). CFU/ml was determined by plating serially-diluted F. nucleatum cultures on Columbia agar plates containing 5% sheep blood and counting colonies after incubating the plates under anaerobic conditions at 37 °C for 2 days.
The ATCC RAW 264.7 mouse macrophage cell line was kindly provided by Alok Joglekar (David Baltimore’s lab at California Institute of Technology). The cells were routinely grown in RPMI medium (Life Technology 1699742) supplemented with L-glutamine, 25 mM HEPES, MEM non-essential amino acids (Life Technology 1703183), 10% FBS and 10 U/mL Penicillin-Streptomycin (Thermo-Fisher 15140-122). Cells were passaged every 2 days in fresh media at least 4 consecutive times before beginning each experiment. For each passage, cells were detached using a cell scraper (320 mm Long, 12 mm Blade, CytoOne) followed by a 3-fold dilution in fresh media.
C. albicans and F. nucleatum co-cultivation morphology and viability
Co-cultivation assays were performed under C. albicans hyphae-inducing conditions as described above. C. albicans yeast cells from an overnight culture and exponential-phase F. nucleatum were harvested and resuspended in fresh YPD plus 20% FBS to achieve ≈1 × 107 CFU/ml and ≈1 × 109 CFU/ml, respectively. We used 100 times more F. nucleatum than C. albicans because this ratio gave us the most inhibited yeast form of C. albicans (~90% yeast). An equal volume of C. albicans and F. nucleatum were mixed and incubated at 37 °C under aerobic conditions for 4 hrs. C. albicans morphology was assessed using light microscopy before and after the 4-hour incubation.
Due to the tendency of C. albicans hyphae to form clumps, a sonication method was applied to disrupt C. albicans aggregates as well as C. albicans/F. nucleatum association in this study. Although it can not achieve total separation, mild sonication is still a relatively effective and widely used method for disrupting C. albicans clumps as well as physical association between C. albicans and other bacteria before plating for colony-forming unit determination22,48,49,50. Briefly, mixed cultures were subjected to mild sonication on ice for 5 cycles of 15 seconds on and 30 seconds off, at power setting 3 using the Sonic Dismembrator Model F60 (Fisher Scientific). The sonicated solution was then serially diluted and plated on both antibiotic-containing YPD agar plates (for enumeration of C. albicans CFU) and Columbia blood agar plates (for enumeration of CFU of C. albicans and F. nucleatum based on their distinct colony morphologies). CFU/ml was calculated after a 2-day incubation under aerobic and anaerobic conditions, respectively.
To assess the effect of co-cultivation with F. nucleatum on the viability of hyphal C. albicans, the hyphal form was prepared under hyphae-inducing conditions. Hyphal C. albicans cell number was determined by subjecting the culture to sonication as described above and performing cell number counting using a hemocytometer as previously reported51. F. nucleatum cells were then added at a 100:1 ratio. The co-culture was further incubated aerobically at 37 °C for 4 hrs. Samples were taken and observed under a light microscope before and after incubation. Viability of C. albicans was determined as described above.
Two-chamber assay
Yeast cells of C. albicans SN152 wild type or the flo9 mutant and F. nucleatum wild type strain ATCC 23726 or the radD mutant were grown to exponential phase. Cells were collected and resuspended in YPD supplemented with 20% FBS. The two-chamber assay was set up as follows: A 2 mL suspension of F. nucleatum wild type alone, or the co-culture of F. nucleatum wild type with C. albicans wild type or flo9 mutant, or co-culture of F. nucleatum radD and C. albicans wild type in a 1:1 volume ratio (F. nucleatum at ≈5 × 108 CFU/ml, C. albicans at ≈5 × 106 CFU/ml) was added to the lower chamber of a 12-well plate containing a 0.4-μm PET membrane insert (Millipore). Subsequently, a 1 mL suspension of C. albicans wild type or flo9 mutant was added to the upper chamber. After a 4-hour incubation at 37 °C under aerobic conditions, samples from the upper and lower chamber were taken and observed using a light microscope. Viability of C. albicans and F. nucleatum from both chambers was determined as described above. All assays were performed in duplicate and repeated three times on different days.
Cytokine and C. albicans viability assays
2 × 106 RAW 264.7 macrophage cells, as determined by hemocytometer cell counting, were seeded in 12-well cell culture plates (Costar 3513) in 1 mL fully supplemented RPMI medium. Cells were incubated overnight to facilitate adherence to the bottom of the plates (100% confluence). The adherent RAW cells were then gently washed with RPMI media supplemented with all components (see above) except penicillin-streptomycin.
The yeast and hyphal C. albicans were prepared as described above. F. nucleatum was incubated with C. albicans for 4 hours before inoculation into the macrophage cell culture. Cell numbers in individual as well as mixed cultures were enumerated on a hemocytometer and washed with RPMI without antibiotics. The yeast or hyphal C. albicans was inoculated into the RAW cell culture at an MOI (multiplicity of infection) of 1:1. After testing multiple different combinations, 1:1 ratio was chosen because this ratio was easy to work with and showed the most drastic difference in viability. Co-cultures of yeast or hyphal C. albicans and F. nucleatum were co-inoculated into the RAW cells at an MOI of 100:1:1 (F. nucleatum: C. albicans: RAW). The culture was then centrifuged at 900 × g for 3 min and incubated at 37 °C for 4 hours in a humidified tissue culture chamber. After incubation, the supernatants were collected and frozen for later use. For cytokine quantification, the supernatants were thawed and centrifuged at 17,000 × g for 5 minutes to remove cell particles and debris. Monocyte Chemotactic Protein 1 (MCP-1) and Tumor Necrosis Factor-α (TNFα) were quantified by a plate-based ELISA assay following the manufacturer’s protocol (Raybiotech). All assays were performed three times on different days.
To assess the viability of C. albicans and F. nucleatum, the mixed bacterial-fungal culture was added to surface-adhered RAW cells or empty wells and centrifuged at 900 × g for 3 min, then incubated at 37 °C for 90 minutes. Co-cultured cells were detached from the plate and washed two times with sterile water to lyse the macrophages. C. albicans and F. nucleatum before and after incubation with RAW cells were subjected to mild sonication as describe above before plating on blood agar plates and incubated under anaerobic conditions for 2 days. CFU/ml of C. albicans and F. nucleatum was determined based on distinct colony morphology. Cell viability was expressed as the percentage of viable cells 90 minutes after incubation compared to the starting cell number. To ensure that fungal and bacterial viability was not significantly affected by washing with water, C. albicans and F. nucleatum alone were also washed with sterile water and plated. All assays were performed in triplicates and repeated three times on different days.
Macrophage viability assay
1.3 × 106 RAW macrophage cells were seeded in 12-well plates as described above. Subsequently, adherent cells were washed with RPMI media without antibiotics. C. albicans and F. nucleatum were prepared as above but with the addition of propodium iodide (PI, 2000:1 dilution) to the RPMI media. C. albicans and F. nucleatum were added to the RAW cells at a 1:10 and 1:1000 ratio respectively. Cells were centrifuged at 900 × g for 3 minutes and incubated for 2, 4 and 6 hours before imaging. Incorporation of PI into the RAW cells was used as an indicator of a compromised cell membrane and was visualized with epifluorescence by a NIKON Eclipse TE200 inverted microscope equipped with a 10x objective. All assays were repeated three times on different days and representative images are shown.
Statistical Analysis
Microsoft Excel was used to perform statistical analyses. Student’s t-test was used to compare variables. A P value of <0.05 denotes significant difference.
Additional Information
How to cite this article: Bor, B. et al. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 6, 27956; doi: 10.1038/srep27956 (2016).
References
Aas, J. A., Paster, B. J., Stokes, L. N., Olsen, I. & Dewhirst, F. E. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43, 5721–32 (2005).
Kuramitsu, H. K., He, X., Lux, R., Anderson, M. H. & Shi, W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71, 653–70 (2007).
Paster, B. J., Olsen, I., Aas, J. A. & Dewhirst, F. E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42, 80–7 (2006).
Ghannoum, M. A. et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6, e1000713, doi: 10.1371/journal.ppat.1000713(2010).
Huffnagle, G. B. & Noverr, M. C. The emerging world of the fungal microbiome. Trends Microbiol 21, 334–41 (2013).
Morales, D. K. & Hogan, D. A. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog 6, e1000886, doi: 10.1371/journal.ppat.1000886 (2010).
Azie, N. et al. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 73, 293–300 (2012).
Coleman, D. C. et al. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS 11, 557–67 (1997).
Perlroth, J., Choi, B. & Spellberg, B. Nosocomial fungal infections: epidemiology, diagnosis and treatment. Med Mycol 45, 321–46 (2007).
Shirtliff, M. E., Peters, B. M. & Jabra-Rizk, M. A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 299, 1–8 (2009).
Carlson, E. & Johnson, G. Protection by Candida albicans of Staphylococcus aureus in the establishment of dual infection in mice. Infect Immun 50, 655–9 (1985).
Peters, B. M. et al. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol 59, 493–503 (2010).
Diaz, P. I. et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun 80, 620–32 (2012).
Xu, H. et al. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol 16, 214–31 (2014).
Strus, M. et al. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect Dis Obstet Gynecol 13, 69–75 (2005).
Peleg, A. Y., Hogan, D. A. & Mylonakis, E. Medically important bacterial-fungal interactions. Nat Rev Microbiol 8, 340–9 (2010).
Signat, B., Roques, C., Poulet, P. & Duffaut, D. Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol 13, 25–36 (2011).
Kolenbrander, P. E. Oral microbial communities: biofilms, interactions and genetic systems. Annu Rev Microbiol 54, 413–37 (2000).
Rickard, A. H., Gilbert, P., High, N. J., Kolenbrander, P. E. & Handley, P. S. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 11, 94–100 (2003).
Grimaudo, N. J. & Nesbitt, W. E. Coaggregation of Candida albicans with oral Fusobacterium species. Oral Microbiol Immunol 12, 168–73 (1997).
Jabra-Rizk, M. A. et al. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. Journal of Clinical Microbiology 37, 1464–8 (1999).
Wu, T. et al. Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum. J Dent Res, doi: 10.1177/0022034515593706 (2015).
He, X., McLean, J. S., Guo, L., Lux, R. & Shi, W. The social structure of microbial community involved in colonization resistance. ISME J 8, 564–574 (2014).
He, X. et al. Oral-derived bacterial flora defends its domain by recognizing and killing intruders–a molecular analysis using Escherichia coli as a model intestinal bacterium. Microb Ecol 60, 655–64 (2010).
Marcil, A., Harcus, D., Thomas, D. Y. & Whiteway, M. Candida albicans killing by RAW 264.7 mouse macrophage cells: effects of Candida genotype, infection ratios and gamma interferon treatment. Infect Immun 70, 6319–29 (2002).
Kaplan, C. W. et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 78, 4773–8 (2010).
O’Donnell, L. E. et al. Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS Yeast Res 15, doi: 10.1093/femsyr/fov077 (2015).
Gow, N. A., van de Veerdonk, F. L., Brown, A. J. & Netea, M. G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10, 112–22 (2012).
Boon, C. et al. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J 2, 27–36 (2008).
Wang, L. H. et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51, 903–12 (2004).
Tampakakis, E., Peleg, A. Y. & Mylonakis, E. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica serovar Typhimurium. Eukaryot Cell 8, 732–7 (2009).
Hogan, D. A. & Kolter, R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296, 2229–32 (2002).
Cruz, M. R., Graham, C. E., Gagliano, B. C., Lorenz, M. C. & Garsin, D. A. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun 81, 189–200 (2013).
Hogan, D. A., Vik, A. & Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54, 1212–23 (2004).
Gibson, J., Sood, A. & Hogan, D. A. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl Environ Microbiol 75, 504–13 (2009).
Kim, Y. & Mylonakis, E. Killing of Candida albicans filaments by Salmonella enterica serovar Typhimurium is mediated by sopB effectors, parts of a type III secretion system. Eukaryot Cell 10, 782–90 (2011).
Krysan, D. J., Sutterwala, F. S. & Wellington, M. Catching fire: Candida albicans, macrophages and pyroptosis. PLoS Pathog 10, e1004139, doi: 10.1371/journal.ppat.1004139 (2014).
van der Graaf, C. A., Netea, M. G., Verschueren, I., van der Meer, J. W. & Kullberg, B. J. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun 73, 7458–64 (2005).
Levitz, S. M. Innate recognition of fungal cell walls. PLoS Pathog 6, e1000758, doi: 10.1371/journal.ppat.1000758 (2010).
Torosantucci, A., Chiani, P. & Cassone, A. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the beta-1, 6 glucan of the fungal cell wall. J Leukoc Biol 68, 923–32 (2000).
Hashimoto, T. In vitro study of contact-mediated killing of Candida albicans hyphae by activated murine peritoneal macrophages in a serum-free medium. Infect Immun 59, 3555–61 (1991).
Blasi, E. et al. Differential susceptibility of yeast and hyphal forms of Candida albicans to macrophage-derived nitrogen-containing compounds. Infect Immun 63, 1806–9 (1995).
Deshmane, S. L., Kremlev, S., Amini, S. & Sawaya, B. E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29, 313–26 (2009).
Aggarwal, B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3, 745–56 (2003).
Park, S. R. et al. Diverse Toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect Immun 82, 1914–20 (2014).
Garsin, D. A. & Lorenz, M. C. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microbes 4, 409–15 (2013).
Kaplan, C. W., Lux, R., Haake, S. K. & Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol 71, 35–47 (2009).
Lu, Y., Su, C., Wang, A. & Liu, H. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol 9, e1001105, doi: 10.1371/journal.pbio.1001105 (2011).
Nett, J. E., Marchillo, K., Spiegel, C. A. & Andes, D. R. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 78, 3650–9 (2010).
Nobile, C. J. et al. A histone deacetylase complex mediates biofilm dispersal and drug resistance in Candida albicans. MBio 5, e01201–14 (2014).
Uppuluri, P. et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 6, e1000828, doi: 10.1371/journal.ppat.1000828 (2010).
Acknowledgements
This work was supported in part by grants from the National Institute of Health (NIH-1-R01-DE020102 and NIH-1-R01-DE023810), National Institute of Health National Research Service Award Postdoctoral Fellowship F32DE025548-01 (B.B.) and NIDCR T90 (DE022734) training award to (M.A.).
Author information
Authors and Affiliations
Contributions
X.H. and W.S. designed the experiments; B.B. and L.C. performed the experiments; B.B., M.A., X.H. and W.S. performed data analysis and prepared Figures; All authors reviewed the manuscript.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bor, B., Cen, L., Agnello, M. et al. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci Rep 6, 27956 (2016). https://doi.org/10.1038/srep27956
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27956
This article is cited by
-
The oral microbiome: diversity, biogeography and human health
Nature Reviews Microbiology (2024)
-
Understanding the characteristics of the host genome and microbiome interaction in oral squamous cell carcinoma: a narrative review
Beni-Suef University Journal of Basic and Applied Sciences (2022)
-
Trans-cellular tunnels induced by the fungal pathogen Candida albicans facilitate invasion through successive epithelial cells without host damage
Nature Communications (2022)
-
Fusobacterium nucleatum and oral cancer: a critical review
BMC Cancer (2021)
-
Biofilm growth and IL-8 & TNF-α-inducing properties of Candida albicans in the presence of oral gram-positive and gram-negative bacteria
BMC Microbiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.