Abstract
Vascular endothelial growth factor-A is a major player in vascular development and a potent vascular permeability factor under physiological and pathological conditions by binding to a decoy receptor Flt1 and its primary receptor Flk1. In this study, we show that Flt1 heterozygous (Flt1+/−) mouse embryos grow up to adult without life-threatening abnormalities but exhibit a transient embryonic edema around the nuchal and back regions, which is reminiscent of increased nuchal translucency in human fetuses. Vascular permeability is enhanced and an intricate infolding of the plasma membrane and huge vesicle-like structures are seen in Flt1+/− capillary endothelial cells. Flk1 tyrosine phosphorylation is elevated in Flt1+/− embryos, but Flk1 heterozygosity does not suppress embryonic edema caused by Flt1 heterozygosity. When Flt1 mutants are crossed with Aspp1−/− mice which exhibit a transient embryonic edema with delayed formation and dysfunction of lymphatic vessels, only 5.7% of Flt1+/−; Aspp1−/− mice survive, compared to expected ratio (25%). Our results demonstrate that Flt1 heterozygosity causes a transient embryonic edema and can be a risk factor for embryonic lethality in combination with other mutations causing non-lethal vascular phenotype.
Similar content being viewed by others
Introduction
Vascular endothelial growth factor (VEGF)-A is a major player in all aspects of vascular development and is also a potent vascular permeability factor that is involved in fluid homeostasis under physiological and pathological conditions1,2,3. VEGF-A works through receptor tyrosine kinases expressed in endothelial cells, Flt1 and Flk1, which are also called VEGFR1 and VEGFR2, respectively4. Gene-targeting studies established that Flt1 serves mainly as a decoy receptor to sequester VEGF-A from activating the other primary receptor Flk1 in vascular development5,6,7,8. Interestingly, VEGF-A haploinsufficiency in mice resulted in embryonic lethality associated with defective blood vessel development9,10, whereas a hypermorphic allele of VEGF-A gene produced severe abnormalities in heart development and embryonic lethality11, indicating that a subtle change of VEGF-A protein levels affects vascular function during mouse embryogenesis. Although local concentration of VEGF-A protein is regulated by a decoy receptor Flt1, it has not been addressed whether Flt1 heterozygosity affects embryonic development.
The lymphatic vascular system functions in concert with the blood vascular system to regulate the tissue fluid homeostasis of the body. Most of the extravasated interstitial fluid is absorbed back into the blood capillaries, whereas the remaining fluid and macromolecules are taken up and transported to venous circulation by lymphatic vessels. Dysfunction or impaired development of lymphatic vessels causes lymphedema12,13,14. We previously reported that loss of Aspp1, a p53-binding protein predominantly expressed in endothelial cells, causes embryonic edema with defective lymphatic development in mice15,16,17.
In this study, we investigated Flt1 heterozygous (Flt1+/−) mice to determine the regulatory role of Flt1 in vascular development and fluid homeostasis since the homozygotes are embryonic lethal with a defect in early blood vessel development5. Here, we show that Flt1 heterozygosity causes embryonic edema with enhanced vascular permeability. We also show that Flt1 heterozygosity can be a risk factor for embryonic lethality in combination with other mutations causing non-lethal vascular phenotype.
Results and Discussion
Flt1+/− mice showed a transient embryonic edema without overt defects in cardiovascular development
Of 263 embryos from mating wild-type (WT) female with Flt1+/− male mice, the expected ratio of genotypes was observed at embryonic day (E) 14.5 (WT 49.0%, Flt1+/− 51.0%). We found that Flt1+/− embryos exhibit edema around the nuchal and back regions from E13.5 till E17.5, compared to WT embryos (Fig. 1A–C, data not shown), while edema resolves by birth. Western blot analysis using embryonic back skin shows an increased level of Flk1 tyrosine phosphorylation in Flt1+/− embryos (Fig. 1D,E). On the other hand, when we analyzed mice harboring a Flt1TK mutant allele lacking the tyrosine kinase domain but bearing an intact extracellular domain for VEGF-A sequestration7, edema was not detected in Flt1+/TK or Flt1TK/TK embryos (Supplementary Fig. S1). These results indicate that reduction in VEGF-A sequestration rather than Flt1 signals affects fluid homeostasis in mouse embryos.
Flt1+/− mice showed a transient embryonic edema without overt defects in vascular development.
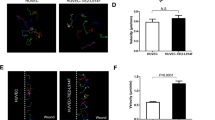
(A,B) Lateral view of whole-mount embryos at E14.5 from crosses between WT and Flt1+/− mice. Subcutaneous edema (arrowheads) is observed only in Flt1+/− embryos. (C) The edema index of Flt1+/− embryos is significantly higher than WT embryos (WT, n = 21; Flt1+/−, n = 16). (D,E) Western blot analysis using embryonic back skin at E14.5 shows the levels of Flk1 tyrosine phosphorylation are elevated in Flt1+/− embryos, compared to WT embryos (n = 3 each group). (F–K) Immunofluorescence confocal microscopic images of flat-mount embryonic back skin at E15.5 stained for PECAM-1 (red) and Flt4 (green). (L) Number of capillary branching points (per mm2) (n = 3 each group). (M) Blood vessel density (vessel area/total area of interest). There was no significant difference in blood and lymphatic vascular network between WT and Flt1+/− embryos (n = 3 each group). Data are presented as mean ± standard error of the mean. ***P < 0.001 and *P < 0.05 as determined by unpaired two-tailed Student’s t-test. N.S. = not significant.
To clarify the causes of embryonic edema in Flt1+/− embryos, we investigated cardiovascular development. Expression analysis using mice carrying a β-galactosidase reporter knocked into the Flt1 or Flk1 locus and blood endothelial cells (BECs) and lymphatic endothelial cells (LECs) isolated from embryonic back skin showed that Flt1 is expressed in BECs but not in LECs, whereas Flk1 is expressed in both endothelial cell types (Supplementary Fig. S2). Whole-mount immunostaining of the back skin for a pan-endothelial marker PECAM-1 and a LEC marker Flt4 (also called VEGFR3) showed that network formation of blood and lymphatic vessels is comparable between WT and Flt1+/− embryos at E15.5 (Fig. 1F–M and Supplementary Fig. S3). The compact layers of ventricles, trabeculation and interventricular septum that are defective in VEGF-A-overexpressing embryos11 are also normal in Flt1+/− embryos (Supplementary Fig. S4).
Flt1+/− embryos showed enhancement of vascular permeability with huge vesicle-like structures in capillary endothelial cells
To look further for the causes of embryonic edema in Flt1+/− embryos, we next investigated vascular permeability by assessing the leakage of Hoechst 33258 injected into blood vessels of embryos from crosses between WT and Flt1+/− mice. Nuclei of BECs alone were stained in WT embryos, whereas those of tissues surrounding blood vessels were stained by Hoechst 33258 leaked out of blood vessels in Flt1+/− embryos, indicating enhancement of vascular permeability (Fig. 2A–C). Since edema in Flt1+/− mice is transient during mid-gestation and is not detected after birth, we performed vascular permeability in adult mice by assessing the leakage of intravenously-injected Evans blue dye after topical application of mustard oil. This analysis showed that vascular permeability is comparable between WT and Flt1+/− adult mice (Supplementary Fig. S5), suggesting that the enhancement of vascular permeability is restricted to embryonic development in Flt1+/− mice.
Flt1+/− embryos showed enhancement of vascular permeability with huge vesicle-like structures in capillary endothelial cells.
(A–C) Vascular permeability assay in E14.5 embryos. Frozen sagittal sections show the nuclei of BECs alone in WT embryos and those of surrounding tissues in Flt1+/− embryos following intravenous injection of Hoechst 33258 (n = 6 each group). (D–J) Transmission electron microscopic images of capillaries in WT and Flt1+/− embryonic back skin at E14.5. Endothelial cells of the blood capillaries containing erythrocytes (e) have an intricate intricate infolding of the plasma membrane and more huge vesicle-like structures (asterisks) which sometimes reach the lateral plasma membrane where adherens junctions reside (H, arrowheads) or are connected to the vascular lumen ((I,J) arrow in adjacent sections) in Flt1+/− embryos. (K) Number of vesicle-like structures in capillary endothelial cells (n = 6 each group). Data are presented as mean ± standard error of the mean. ***P < 0.001 and **P < 0.01 as determined by unpaired two-tailed Student’s t-test. N.S. = not significant.
It has been shown that VEGF-A induces vascular permeability by regulating paracellular and transcellular transports in endothelial cells18. VEGF-A activates Src pathway leading to loosening of endothelial cell-to-cell junctions19,20,21,22 and an in vivo injection of VEGF-A induces the formation of vesiculo-vacuolar organelles23. To address the cellular basis of enhanced vascular permeability in Flt1+/− embryos, we investigated VE-cadherin, Claudin-5 and β-catenin proteins, components of adherens junction or tight junction, but their staining patterns do not appear different between WT and Flt1+/− embryos (Supplementary Fig. S6). Western blot analysis using embryonic back skin showed that Src activity assessed by Y416 phosphorylation (Src-pY416) is comparable between WT and Flt1+/− embryos (Supplementary Fig. S7). Loss of pericyte coverage is also known to correlate with enhanced vascular permeability24,25,26, but immunostaining of a pericyte marker Desmin together with PECAM-1 showed that pericyte coverage of capillary blood vessels is not affected in Flt1+/− embryos (Supplementary Fig. S8). Transmission electron microscopic analysis showed an intricate infolding of the plasma membrane and more huge vesicle-like structures in Flt1+/− blood capillary endothelial cells (Fig. 2D–K). Vesicle-like structures sometimes reach the lateral plasma membrane where adherens junctions reside or are connected to the vascular lumen in Flt1+/− embryos (Fig. 2H–J). These morphological changes in endothelial cells are most likely related to the enhanced vascular permeability in Flt1+/− embryos although it remains to be elucidated whether paracellular and/or transcellular transports are involved.
Flk1 heterozygosity does not suppress embryonic edema caused by Flt1 heterozygosity
Flk1 is the primary receptor for VEGF-A and its tyrosine phosphorylation is elevated in Flt1+/− embryos. To investigate whether Flk1 heterozygosity suppresses embryonic edema caused by Flt1 heterozygosity, we crossed Flt1+/− and Flk1+/− mice. Flt1+/−; Flk1+/− and Flt1+/− embryos exhibited a comparable edema although Flk1+/− embryos appeared normal (Supplementary Fig. S9). In addition to embryonic edema, Flt1+/−; Flk1+/− mice surprisingly showed buphthalmia caused by abnormal aqueous humor homeostasis, as we reported previously27. Although Flk1+/− mice do not exhibit embryonic edema or buphthalmia, it will be intriguing to look further into subtle vascular defects of Flk1+/− mice in the future.
Flt1 heterozygosity promotes death of Aspp1−/− embryos
We previously reported that Aspp1−/− mice exhibit a transient embryonic edema with delayed formation and dysfunction of lymphatic vessels in embryos. The edema resolves by birth and no overt abnormalities have been detected in adults17. Similarly, Flt1+/− mice grow up normally to adult without life-threatening abnormalities, despite a transient embryonic edema caused by enhanced vascular permeability. To test whether dysregulation of fluid homeostasis may influence the viability of mouse embryos, we crossed these two mutants and analyzed occurrence and extent of edema, vascular formation, vascular permeability and survival rate of their offspring. When we crossed Flt1+/−; Aspp1+/− mice and Aspp1−/− mice, all embryos except Aspp1+/− embryos showed edema at E14.5 (Fig. 3A–D,M). Flt1 heterozygosity greatly enhanced edema in Aspp1−/− embryos. Moreover, only 5.7% of all offspring was Flt1+/−; Aspp1−/− at weaning, which is significantly lower than the predicted Mendelian frequency (25%) (Table 1A). We did not notice any loss of pups during the postnatal periods between birth and weaning, suggesting that loss of Flt1+/−; Aspp1−/− mice during embryogenesis is critical. Whole-mount immunostaining of the back skin for PECAM-1 and Flt4 showed that partly-distended lymphatic vessels and lymphatic islands, both of which are a characteristic of Aspp1−/− embryos, are detected in mice carrying the Aspp1−/− mutation (Fig. 3E–H). On the other hand, vascular permeability assessed by Hoechst 33258 injection is enhanced in mice carrying the Flt1+/− mutation (Fig. 3I–L,N). When Aspp1 mutants were crossed with Flt1TK mutants, Flt1+/TK; Aspp1−/− mice survived to adult at a normal Mendelian frequency (Table 1B). These results suggest that both lymphatic dysfunction and enhanced vascular permeability are responsible for stronger edema formation and lethality of Flt1+/−; Aspp1−/− embryos. They have been reported that Src plays a role in VEGF-A-induced vascular permeability19,20,21,22 and that ASPP and Src play a cooperative role in epithelial organization in Drosophila28. Western blot analysis using embryonic back skin from mating between Flt1+/−; Aspp1+/− mice and Aspp1−/− mice showed that Src-pY416 is elevated in Flt1+/−; Aspp1−/− mice but not in other littermates including Flt1+/−; Aspp1+/− and Aspp1−/− mice (Fig. 3O,P). This result suggests a synergistic regulation of Src by VEGF-A and Aspp1 although a detailed mechanism remains to be addressed.
Flt1 heterozygosity enhances embryonic edema in Aspp1−/− embryos.
(A–D) Lateral view of whole-mount embryos at E14.5 from crosses between Flt1+/−; Aspp1+/− and Aspp1−/− mice. Subcutaneous edema (arrowheads) is detected in all embryos except Aspp1+/− embryos. Flt1 heterozygosity greatly enhances edema in Aspp1−/− embryos. (E–H) Immunofluorescence confocal microscopy images of flat-mount embryonic back skin at E15.5 stained for PECAM-1 (red) and Flt4 (green). Partly-distended lymphatic vessels and lymphatic islands (arrowheads) are detected in mice carrying Aspp1−/− mutation. (I–L) Vascular permeability assay in E14.5 embryos. Frozen sagittal sections show the nuclei of surrounding tissues as well as BECs in mice carrying Flt1+/− but not Aspp1−/− mutation following intravenous injection of Hoechst 33258. (M) The edema index of Flt1+/−; Aspp1+/−, Aspp1−/− and Flt1+/−; Aspp1−/− embryos are significantly higher than Aspp1+/− embryos (Aspp1+/−, n = 6; Flt1+/−; Aspp1+/−, n = 4; Aspp1−/−, n = 8; Flt1+/−; Aspp1−/−, n = 3). (N) Vascular permeability is enhanced with mice carrying Flt1+/− mutation (n = 3 each group). (O,P) Western blot analysis using embryonic back skin at E14.5 shows that Src activity is elevated in Flt1+/−; Aspp1−/− mice but not in other littermates (n = 3 each group). Data are presented as mean ± standard error of the mean. ***P < 0.001, **P < 0.01 and *P < 0.05 as determined by one-way analysis of variance for inter-group comparisons and Tukey-Kramer method for group comparisons. N.S. = not significant.
In contrast to a reduction of Flt1 causing a transient embryonic edema, it has been reported that elevated levels of circulating soluble Flt1 are associated with preeclampsia in humans, a severe complication of pregnancy involving maternal hypertension, proteinuria and glomerular malfunction29. Taken together, a fine tuning of fluid homeostasis by a balance between VEGF-A and decoy receptor Flt1 is crucial for proper embryonic development and maternal health. These results also suggest that alterations in Aspp1 or Flt1 function could underlie the transient increase in nuchal translucency observed in some human fetuses, a defect that can still be compatible with a normal pregnancy outcome30,31. However, combined defects in pathways affecting vascular permeability and lymphatic drainage could lead to fetal loss.
Materials and Methods
Mice
A colony of mice heterozygous for Flt1+/lacZ, Flk1+/lacZ, Flt1+/TK, or Aspp1+/lacZ was maintained as described previously7,17,32. Noon of the day on which the vaginal plug was detected was considered as E0.5. Embryos were genotyped by PCR analysis of tail DNA. Animal experiments were approved by the Institution Animal Care and Use Committee of Kobe University Graduate School of Medicine and carried out in accordance with the animal experimentation guidelines of the Kobe University Graduate School of Medicine.
Measurement of embryonic edema
Digital images of embryo propers were acquired with illumination from the bottom under dissecting microscope and the number of pixels for a transparent area of embryos and crown-rump length (CRL) was measured. The extent of embryonic edema was examined as an edema index, which is a transparent area divided by the square of CRL.
Western blot analysis
Whole embryos were dissected at E14.5 and the back skin was peeled off and lysed in 50 mmol/L Tris-HCl (pH7.4) containing 1% Triton X-100, 150 mmol/L NaCl, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L phenylmethylsulfonyl fluoride, 20 mmol/L NaF, 10 mmol/L Na4P2O7, 2 mmol/L Na3VO4, protease inhibitor cocktail and phosphatase inhibitor. After 30 min incubation at 4 °C, the lysate was clarified by centrifugation at 10000 g for 15 min at 4 °C. The supernatant containing equal amounts of protein was used as samples for immunoprecipitation, SDS-PAGE and western blot on PVDF membranes with antibodies to Src and Src-pY416. For detection of total and phosphorylated Flk1, Flk1 proteins were collected from the lysate by immunoprecipitation with anti-Flk1 antibody (clone 55B11), followed by western blot with antibodies to Flk1 or phospho-tyrosine (clone PY20). We used horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG as secondary antibodies and enhanced chemiluminescence for detection with LAS-1000 (Fujifilm). To quantitate phosphorylated protein per total protein, the densitometric analysis of digital images was performed using the ImageJ software (National Institutes of Health).
Immunohistochemistry
We used goat anti-Flt4, rat anti-PECAM-1 (clone Mec13.3), Armenian hamster anti-PECAM-1 (clone 2H8), rat anti-LYVE-1 (clone ALY7)33, rabbit anti-β-galactosidase, rat anti-VE-cadherin (clone 11D4.1), mouse anti-Claudin-5 (clone 4C3C2), rabbit anti-β-catenin, goat anti-Flk1 and mouse anti-Desmin (clone D33) antibodies. For immunofluorescence, we used Alexa Fluor 488-, Cy3-, or Cy5-conjugated secondary antibodies.
For immunohistochemical analysis of embryonic back skin, whole embryos were dissected at E14.5 and E15.5 and fixed in phosphate-buffered saline (PBS) containing 4% paraformaldehyde at 4 °C for 10 minutes. The back skin was peeled off and further fixed in the same fixative at 4 °C for 3 hours. Tissues were washed in PBS with 0.2% Triton X-100 (PBT) at 4 °C for 30 minutes twice, blocked in PBT containing 1% bovine serum albumin at room temperature for 1 hour and stained with primary antibodies in blocking solution at 4 °C overnight. Tissues were washed in PBT for 30 minutes 3 times at 4 °C and twice at room temperature, followed by staining with secondary antibodies in blocking solution at 4 °C overnight. Tissues were washed in PBT for 30 minutes 3 times at 4 °C and twice at room temperature. Back skin was flat-mounted on slide glasses in ProLong Antifade and analyzed by confocal laser scanning microscopy (FV-1000, Olympus or LSM 700, Zeiss). Quantification of pericyte coverage was measured on the number of pixels of PECAM-1+ blood vessels and Desmin+ pericytes.
Vascular permeability assay
Embryos were recovered with intact yolk sac and placenta at E14.5, perfused individually with Hoechst 33258 (2 μg/embryo) from the vitelline vein for 5 minutes and dissected further to collect the embryo propers. The embryos were mounted in OCT compound and cut sagittally in 10 μm thick cryosections. The distribution of stained nuclei was examined by inverted fluorescent microscopy (IX81, Olympus). The extent of vascular permeability of dermal blood vessels was measured as the number of stained nuclei per total cell number in a circular area of 20000 μm2.
Adult mice at 6 to 7 weeks of age were anesthetized and injected with Evans blue dye (30 mg/kg in 300 μl) into a tail vein. After 1 minute, 5% mustard oil in mineral oil was applied to the dorsal and ventral surfaces of the ear with a cotton tip. After 30 minutes, mice were perfused with 10 ml of PBS containing 1% paraformaldehyde to wash out dye from blood vessels. Ears were removed, dried at 55 °C overnight and weighed. Evans blue was extracted from the ears in 1 ml of formamide at 55 °C overnight and measured with spectrophotometry at OD610. The standard curve was prepared from serial dilutions of Evans blue dye at known concentration in formamide and used to determine the concentration in samples. The extent of vascular permeability was measured as the weight of extracted dye per weight of ear34. We analyzed one ear per mouse.
Transmission electron microscopic analysis
Trunk of embryos was fixed in 0.1 M cacodylate buffer (pH7.4) containing 2% paraformaldehyde and 2% glutaraldehyde at room temperature for 2 hours and at 4 °C overnight. After the fixation, the samples were postfixed in 1% osmium tetroxide in the same buffer for 1 hour on ice, dehydrated in a series of graded ethanol and embedded in resin. Thin sections were cut with an ultramicrotome (ULTRACUT, Leica) and examined in an electron microscope (JEM-1010, JEOL Ltd.).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated by an RNeasy Mini Kit and RNase-Free DNase Set from PECAM-1+/LYVE-1−/CD45− BECs and PECAM-1+/LYVE-1+/CD45− LECs purified from the back skin at E14.5, as described previously17. First-strand cDNA was synthesized using the Super Script first-strand synthesis system for RT-PCR according to the manufacturer’s instructions and used for PCR analysis with specific primers for Flt1 (5′-TGTGGAGAAACTTGGTGACCT-3′ and 5′-TGGAGAACAGCAGGACTCCTT-3′), Flk1 (5′-AGAACACCAAAAGAGAGGAACG-3′ and 5′-GCACACAGGCAGAAACCAGTAG-3′), PECAM-1 (5′-CAAACCGTATCTCCAAAGCC-3′ and 5′-TCTGTGAATGTTGCTGGGTC-3′), LYVE-1 (5’-TTCCTCGCCTCTATTTGGAC-3′ and 5′-ACGGGGTAAAATGTGGTAAC-3′), Prox1 (5′-AAGTGGTTCAGCAATTTCCG-3′ and 5′-TGACCTTGTAAATGGCCTTC-3′) and β-actin (5′-GGACTCCTATGTGGGTGACGAGG-3′ and 5′-GGGAGAGCATAGCCCTCGTAGAT-3′).
Hematoxylin and Eosin (H&E) staining
E14.5 embryos were fixed in PBS containing 4% paraformaldehyde at 4 °C overnight, dehydrated into methanol, embedded in paraffin, cut in 5 μm thick sections and stained with H&E by standard procedures.
Statistical analysis
Values are presented as mean ± standard error of the mean. The unpaired two-tailed Student’s t-test was used to compare the results between two groups. One-way analysis of variance was used for inter-group comparisons and Tukey-Kramer method was used for group comparisons. Chi-square test was performed to compare the actual survival to expected numbers. At least three specimens were analyzed in all cases. P-values are indicated as follows; ***P < 0.001, **P < 0.01 and *P < 0.05.
Additional Information
How to cite this article: Otowa, Y. et al. Flt1/VEGFR1 heterozygosity causes transient embryonic edema. Sci. Rep. 6, 27186; doi: 10.1038/srep27186 (2016).
References
Keck, P. J. et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246, 1309–1312 (1989).
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Bates, D. O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 87, 262–271, doi: 10.1093/cvr/cvq105 (2010).
Adams, R. H. & Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478, doi: 10.1038/nrm2183 (2007).
Fong, G. H., Rossant, J., Gertsenstein, M. & Breitman, M. L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376, 66–70, doi: 10.1038/376066a0 (1995).
Shalaby, F. et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66, doi: 10.1038/376062a0 (1995).
Hiratsuka, S., Minowa, O., Kuno, J., Noda, T. & Shibuya, M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA 95, 9349–9354, doi: 10.1073/pnas.95.16.9349 (1998).
Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 39, 469–478 (2006).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439, doi: 10.1038/380435a0 (1996).
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442, doi: 10.1038/380439a0 (1996).
Miquerol, L., Langille, B. L. & Nagy, A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 127, 3941–3946 (2000).
Norrmen, C., Tammela, T., Petrova, T. V. & Alitalo, K. Biological basis of therapeutic lymphangiogenesis. Circulation 123, 1335–1351, doi: 10.1161/CIRCULATIONAHA.107.704098 (2011).
Tammela, T. & Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476, doi: 10.1016/j.cell.2010.01.045 (2010).
Yang, Y. & Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Invest. 124, 888–897, doi: 10.1172/JCI71609 (2014).
Trigiante, G. & Lu, X. ASPP and cancer. Nat. Rev. Cancer 6, 217–226, doi: 10.1038/nrc1818 (2006).
Hirashima, M., Bernstein, A., Stanford, W. L. & Rossant, J. Gene-trap expression screening to identify endothelial-specific genes. Blood 104, 711–718, doi: 10.1182/blood-2004-01-0254 (2004).
Hirashima, M. et al. Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Dev. Biol. 316, 149–159, doi: 10.1016/j.ydbio.2008.01.023 (2008).
Weis, S. M. & Cheresh, D. A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437, 497–504, doi: 10.1038/nature03987 (2005).
Antonetti, D. A., Barber, A. J., Hollinger, L. A., Wolpert, E. B. & Gardner, T. W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 274, 23463–23467 (1999).
Eliceiri, B. P. et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 (1999).
Pedram, A., Razandi, M. & Levin, E. R. Deciphering vascular endothelial cell growth factor/vascular permeability factor signaling to vascular permeability. Inhibition by atrial natriuretic peptide. J. Biol. Chem. 277, 44385–44398, doi: 10.1074/jbc.M202391200 (2002).
Sun, Z. et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J. Exp. Med. 209, 1363–1377, doi: 10.1084/jem.20111343 (2012).
Feng, D., Nagy, J. A., Hipp, J., Dvorak, H. F. & Dvorak, A. M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine and serotonin. J. Exp. Med. 183, 1981–1986 (1996).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561, doi: 10.1038/nature09522 (2010).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566, doi: 10.1038/nature09513 (2010).
Hellstrom, M. et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 153, 543–553 (2001).
Sano, K. et al. Flt1 and Flk1 mediate regulation of intraocular pressure and their double heterozygosity causes the buphthalmia in mice. Biochem. Biophys. Res. Commun. 420, 422–427, doi: 10.1016/j.bbrc.2012.03.011 (2012).
Langton, P. F., Colombani, J., Aerne, B. L. & Tapon, N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev. Cell 13, 773–782, doi: 10.1016/j.devcel.2007.11.005 (2007).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658, doi: 10.1172/JCI17189 (2003).
Haak, M. C. & van Vugt, J. M. Pathophysiology of increased nuchal translucency: a review of the literature. Hum. Reprod. Update 9, 175–184 (2003).
Souka, A. P., Von Kaisenberg, C. S., Hyett, J. A., Sonek, J. D. & Nicolaides, K. H. Increased nuchal translucency with normal karyotype. Am. J. Obstet. Gynecol. 192, 1005–1021, doi: 10.1016/j.ajog.2004.12.093 (2005).
Hirashima, M., Lu, Y., Byers, L. & Rossant, J. Trophoblast expression of fms-like tyrosine kinase 1 is not required for the establishment of the maternal-fetal interface in the mouse placenta. Proc. Natl. Acad. Sci. USA 100, 15637–15642, doi: 10.1073/pnas.2635424100 (2003).
Morisada, T. et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 105, 4649–4656, doi: 10.1182/blood-2004-08-3382 (2005).
Thurston, G. et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286, 2511–2514 (1999).
Acknowledgements
We thank Kazuyo Misaki for technical assistance and Masahide Sakabe and Yosuke Fujii for technical advice. This work was supported in part by the Uehara Memorial Foundation (M.H.), the SENSHIN Medical Research Foundation (M.H.), Grant-in-Aid for Scientific Research (C) (M.H., No. 22590173), Grant-in-Aid for Scientific Research on Innovative Areas (M.H., No. 24112513) and the Global COE Program (A08) “Global Center for Education and Research in Integrative Membrane Biology” from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
M.H. conceived and designed the experiments; M.H., T.S. and Y.K. conducted the experiments, Y.O., K.M., K.S., M. Shirakabe and S.Y. performed the experiments and analyzed the data; M. Shibuya and J.R. contributed reagents/materials. All authors reviewed and contributed to the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Otowa, Y., Moriwaki, K., Sano, K. et al. Flt1/VEGFR1 heterozygosity causes transient embryonic edema. Sci Rep 6, 27186 (2016). https://doi.org/10.1038/srep27186
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27186
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.