Abstract
Molybdenum disulfide (MoS2) has been attracting much attentions due to its excellent electrical and optical properties. We report here the synthesis of large-scale and uniform MoS2 nanosheets with vertically standing morphology using chemical vapor deposition method. TEM observations clearly reveal the growth mechanism of these vertical structures. It is suggested that the vertical structures are caused by the compression and extrusion between MoS2 islands. More importantly, the vertical morphology of two dimensional (2D) materials hold many promising potential applications. We demonstrate here the as-synthesized vertically standing MoS2 nanosheets could be used for hydrogen evolution reaction, where the exchange current density is about 70 times of bulk MoS2. The field emission performance of vertically standing MoS2 were also improved due to the abundantly exposed edges.
Similar content being viewed by others
Introduction
Graphene has attracted extensive interests in various research fields since it was obtained through mechanical exfoliation by Novoselov et al. in 20041. Due to the distinctive physical properties of one-layer thin 2D materials compared with their bulk counterparts2,3, layered materials have attracted much attentions, such as transition metal dichalcogenides (TMDCs)4, transition metal oxides5, boron nitride (BN)6, etc. Lots of efforts have been made by using 2D materials in the fields of microelectronics7,8, optoelectronics9, sensors10 and energy storage11,12. However, these works have been devoted to utilize 2D materials lying flat on the substrates. Less of attention has been paid on their alternative configuration13,14,15. Amongst these nanometric architectures, vertically standing 2D materials hold great potential in many applications due to their high aspect ratio and extensively exposed edges16.
For example, the minimized dimension and vertically aligned morphology of 2D materials consequently enable the fabrication of mini-sized energy storage devices with high capacity and high packing density, such as hydrogen storage devices, batteries and supercapacitors. The exposed edges with dangling bonds are chemical active and may play an important role in many catalytic reactions, such as hydrodesulfurization, hydrogen evolution reaction (HER) etc17,18,19,20. J. Shi et al.20 demonstrate that the HER activity relates closely to the edge sites of MoS2 flakes and the basal surfaces are catalytically inert, revealing the importance of exposed edges in catalytic reactions21. Furthermore, It has been demonstrated that vertically growth 1D nanotubes/nanowires and 2D nanosheets with atomically thin edges can significantly improve the field emission properties22,23, making vertically standing 2D materials promising candidates in field emission applications24,25,26.
There have already been lots of works based on the one dimensional (1D) nanowires and nanotubes. And the growth mechanism for vertical 1D nanowires and nanotubes are widely discussed. Normally, nanowires and nanotubes are assumed to be grown at the interface between catalytic and nanowires (nanotubes) via vapor-liquid-solid (VLS) or vapor-solid-solid (VSS) process in 1D growth27,28. In contrast, the growth mechanisms for vertically standing 2D materials are still vague. L. Jiang et al.24 demonstrated that a transition from 2D complete films to 3D clusters beyond a critical layer thickness may be caused by the sufficient accumulation of strain energy and the defects of the as-deposited film during vertical graphene growth process. J. Zhao et al.29 demonstrated that vertically standing graphene could be nucleated from the buffer layer or from the surface of carbon onions. However, there are no clear evidences demonstrating that how these transitions from 2D films to 3D clusters happened. We believe that a clear understanding of the growth mechanism would facilitate the development of vertically standing materials based applications. In addition, the growth mechanism could also promote the process in designing more complex nanometric structures.
In this work, we propose a method to synthesize vertically standing MoS2 nanosheets using a conventional chemical vapor deposition (CVD) method. Various characterizations techniques were used to give a deep analysis of those vertically standing MoS2 nanosheets. In addition, a possible mechanism is proposed based on the experimental results. Furthermore, the field emission properties and the HER performance of vertically standing MoS2 nanosheets were reported.
Results
Synthesis and characterization of vertically standing MoS2
A typical SEM image of the vertically standing MoS2 nanosheets grown on SiO2/Si substrate is shown in Fig. 1a. It clearly shows that MoS2 nanosheets were uniformly grown on the substrate. The 52.5° tilt SEM image (Fig. 1b) demonstrates that the as-grown MoS2 nanosheets are nearly perpendicular to the substrate. AFM height profile is shown in Fig. 1c, where the corresponding AFM image is shown in the inset. The nanometric edges of different MoS2 layers could be clearly observed in the high magnification SEM image (see Supplementary Fig. S1 online). It is believed that these nanometric protrusions and edges are catalytically active sites which could significantly enhance the catalytic and field emission properties.
Characterization of as-synthesized MoS2 nanosheets.
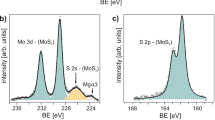
(a) Top view FE-SEM image of vertically standing MoS2 nanosheets. (b) 52.5° tilt view of MoS2 nanosheets which clearly show the vertically standing feature. (c) AFM height profile of MoS2 nanosheets, the insert shows the corresponding AFM image. (d) Cross-sectional TEM image of vertically standing MoS2 grown on SiO2/Si substrate. (e) Region1 (yellow block in d): High-magnification cross-sectional TEM image of MoS2 layers, clearly shows the layered structure of MoS2 nanosheets. (f) Region2 (blue block in d): High-magnification cross-sectional TEM images on the top of vertically standing MoS2 nanosheets and nanometric edge with size of several nanometers could be observed. (g) Raman spectrum of our sample and single-crystal bulk MoS2 obtained by mechanical exfoliation. (h) XPS spectra of Mo 3d and S 2s peaks. (i) XPS spectra of S 2p peak.
To further explore the microstructure and quality of the as-grown MoS2 nanosheets, cross-section TEM analysis were carried out. TEM samples were prepared using Focused Ion Beam (FIB) process. The cross-section TEM image (Fig. 1d) shows the general morphologies of a vertically free standing MoS2 nanosheets grown on SiO2/Si substrate. The synthesized MoS2 nanosheet stands nearly perpendicularly upon the substrate and the height of this MoS2 sheet is about 1.1 μm. High resolution TEM images are shown in Fig. 1e,f, corresponding to the region 1 and 2 marked in Fig. 1d respectively, where layered structure of MoS2 could be clearly observed. The distance between two MoS2 layers was measured to be about 0.65 nm (Fig. 1e), which is consistent with previous reports7,30. The thickness of MoS2 tip was measured to be 3.8 nm, corresponding to 5 layers of MoS2. It’s worth to note that the thickness of MoS2 nanosheet is non-uniform. The thickness of MoS2 increased from 3.8 nm to be 9.4 nm in the middle region of the sheet (Fig. 1e,f), revealing a tapered morphology of MoS2 nanosheets. More morphology TEM images could be found in the Supplementary Fig. S2. The same morphology observation was reported in the free-standing vertical graphene by Zhao et al.29 It’s believed that these pyramid-like shape was caused by the terraces and steps formed during the growth, which also maintain the stability of the vertical structure.
MoS2 nanosheets were also characterized using Raman spectroscopy. A typical Raman spectrum of our CVD grown MoS2 sample is displayed in Fig. 1g. Two Raman characteristic bands at 410 and 384 cm−1 with the full-width-half-maximum (FWHM) values of 5.8 and 5.9 cm−1 could be found, corresponding to the out-of–plane A1g and in-plane E12g vibration of MoS2 respectively30,31,32. Figure 1g also presents the Raman spectrum of a single-crystal bulk MoS2 obtained by mechanical exfoliation as a reference. The similar value of FWHM between CVD samples and pristine MoS2 reveals the high quality of CVD grown MoS2. Note that the Raman peak corresponding to the out-of-plane mode (A1g) is determined by sulfur atoms vibrating along c axis while the in-plane E12g mode are seen to involve motions of molybdenum and sulfur atoms in the basal plane33. Thus it can be inferred that there are more exposed edges in the MoS2 films grown by CVD than those obtained by mechanical exfoliation. Figure 1h,i display detailed XPS spectrum of Mo and S binding energies. The survey scan is provided in Supplementary Fig. S3. None oxidized Mo or S is found after the examination of Mo and S peaks.
Growth mechanism for vertically standing MoS2 nanosheets
To elucidate the growth mechanism of these vertically standing MoS2 nanosheets, different samples with varied growth time were prepared and examined. After 2 minutes growth in 750 °C, many speck-like features could be observed (Fig. 2a) and it is believed that those spots are the nucleation sites for horizontal growth of monolayer MoS2 films. With growth time continued, most seeds grew into larger domain sizes and some seeds merged into one uniform film and some triangular MoS2 islands were formed on top of MoS2 films at the same time (Fig. 2b). More triangle-shaped MoS2 islands were formed above MoS2 films after 5 minutes growth (Fig. 2c), after that, a transformation from 2D growth to 3D growth started (Fig. 2d). Raman spectroscopy analysis have been applied on those samples with different growth time. As shown in Supplementary Fig. S4, the intensity ratios between A1g and E12g modes of CVD grown MoS2 nanosheets samples are higher than that of bulk MoS2 samples, revealing a higher density of exposed edges in those CVD grown MoS2 nanosheets samples. With growth time increased from 5 minutes to 10 minutes, the ratio between A1g and E12g mode intensity was also increased, suggesting a transformation from 2D to 3D growth.
SEM and TEM observation on the formation of vertically standing MoS2 nanosheets.
(a–d) SEM images of the MoS2 films grown at the same condition but with different growth time: (a) 2 minutes, (b) 3 minutes, (c) 5 minutes and (d) 7 minutes. (e–f) SEM observations on the initial stage of the vertical MoS2 formation. (g) Cross-sectional TEM image of vertically standing MoS2. (h) HR-TEM image of plane MoS2 nanosheets. (i) NBD pattern from h (selected area marked by the circle).
More SEM observations were performed on the intermediate state of MoS2 nanosheets to find out how these vertical MoS2 nanosheets are formed. It could be observed in Fig. 2e,f that MoS2 seedlings are originated from the aggregation zone of different MoS2 islands. Thus, it can be inferred from the SEM observations that the formation of vertical structures were caused by the interaction between different MoS2 islands. Cross-section TEM of a vertical MoS2 nanosheet (Fig. 2g) exhibits this nanosheets originates from a thick MoS2 film at the bottom. High resolution TEM image (Fig. 2h) shows the thick MoS2 film has clear layered structure. From the TEM observations, it could be observed that the vertical MoS2 nanosheet are originated form the MoS2 island layers. Based on above results, it could be inferred that vertical standing MoS2 seedlings may originate due to the curling force introduced by the increasing defects and strain energy34. Figure 2i display the nanobeam electron diffraction (NBD) pattern from Fig. 2h (selected area marked by the circle), which shows only regularly arranged diffraction spots, corresponding to the hexagonal crystal structure of MoS2.
TEM analyses were performed on the collision or distortion area where the vertically standing MoS2 nanosheets nucleated from. Basically, two kinds of vertical morphology and their corresponding base structure were observed (Fig. 3). It can be clearly observed in the TEM images that the vertical MoS2 nanosheets were grown from the buffer layer underneath on both cases. The first kind of vertical morphology is shown in Fig. 3a,b. The MoS2 nanosheets are originated from the buffer layers and formed by the extension and the curl of the plane MoS2 buffer layers. Alternatively, the vertical structure could be originated from the merge of two separated MoS2 films (Fig. 3e,f). The formation of vertical morphology may be caused by the collision between two MoS2 islands. The same triangular structure could be clearly observed at the intermediate state as shown in Supplementary Fig. S5. In addition, the stable triangle could be observed on the bottom structure on other TEM observations. Energy-dispersive X-ray spectroscopy (EDX) (see Supplementary Fig. S6 online) certify that nearly no chemical elements are existed in the triangle area, suggesting that the empty triangle was formed due to the curl of MoS2 films instead of other chemical reaction. We believe that the self-formative triangular area could enhance the stability of the whole vertical structure.
Based on the above observations, a vertical growth mechanism is proposed to explain the formation of vertically standing MoS2 nanosheets. It is believed that the intensive compression between different MoS2 islands cause the collision and slide of MoS2 plates, which induce the vertical structure growth. As shown in Fig. 3c,d, when a high barrier of MoS2 islands or plates block the extension of MoS2 islands, the MoS2 islands may slip and change to vertical growth. Alternatively, when two MoS2 island push and collide against each other, the compression force may produce obvious distortion that an arch structure could be formed to release the pressure (Fig. 3g,h), which would subsequently act as the growth templet for the vertical growth of MoS2 nanosheets. Because vertical MoS2 are associated with MoS2 islands, it could be inferred that the alignment of vertically standing MoS2 nanosheets would be improved by synthesizing unanimous MoS2 islands with constant growth direction. MoS2 growth orientation has been reported to be facet-dependent35. Thus, the alignment might be improved by choosing a specific substrate.
To better understand the growth model, schematic graphs are shown in Fig. 4a–e. During the growth period, firstly, MoO3 powder was partially reduced by sulfur vapor to form volatile MoO3−x or gaseous MoS2 and these sub-oxide compounds or gaseous MoS2 were adsorbed and diffused to the substrate and subsequently formed nucleation sites of MoS2 films (Fig. 4a)36. With growth time increased, separated 2D MoS2 films were generally formed due to the growth and merging of the seeds as illustrated in Fig. 4b. It has been shown that the concentration of the gaseous MoS2 or sub-oxide compounds is an important thermodynamics and kinetics factor for the MoS2 growth37. Due to the high concentration of the reactants introduced by the fast evaporation of sulfur powder in our experiment (see Methods), the growth of MoS2 films would be facilitated by the supersaturated MoS2 vapor and sub-oxide compounds vapor. And multi-layer MoS2 films were consequently formed with increasing growth time. After that, MoS2 films are grown in a layer-by-layer fashion until a certain critical thickness (Fig. 4c). And then MoS2 islands were formed (Fig. 4d) to get an energetic favorable morphology according to the Stranski-Krastanov (SK) growth model38,39. With growth continued, MoS2 islands quickly extended and merged. A high strain energy would be introduced due to the fast chemical reaction. Thus, MoS2 seedlings could originate from the collision or distortion area of different MoS2 islands as shown in Fig. 4e. It is worth to note that though vertically standing MoS2 could originate from different based structures, the growth direction and the dominated surface facets remain the same: with  planes defining the two dominant surfaces (see Fig. 4f). The observed morphologies of the MoS2 are driven by the requirement for reducing surface energy. Such a {002}-dominant surface structure is caused by the lowest surface-energy of (002) surface, which is consistent with previous report40.
planes defining the two dominant surfaces (see Fig. 4f). The observed morphologies of the MoS2 are driven by the requirement for reducing surface energy. Such a {002}-dominant surface structure is caused by the lowest surface-energy of (002) surface, which is consistent with previous report40.
Growth model of vertically standing MoS2 nansheets.
(a) “Nucleation stage”: nucleation sites were formed due to the reaction of MoO3−X and S. (b) Two-dimensional MoS2 film was generally formed due to the growth and merge of seeds and bilayer MoS2 sheets were formed due to the continuous supply of reactant. (c) Multi-layer MoS2 films were generally formed. (d) MoS2 islands were formed beyond a certain critical MoS2 layers and their interaction may cause the accumulation of deformation energy. (e) The vertically standing MoS2 nanosheets may formed due to the horizontal and vertical compression and dilatation caused by the regional compression. (f) The simplified model for “vertical MoS2 nanobelts”.
HER and field emission performance
To evaluate the catalytic activity of vertically standing MoS2 nanosheets, a typical three-electrode setup was used for HER test. Vertically standing MoS2 nanosheets were deposited on gold films using the same CVD method as described previously (see Supplementary Fig. S7 online). Typical cathodic polarization curves and corresponding Tafel plots are shown in Fig. 5a,b. The polarization curve of bare gold electrodes is also given in Fig. 5a. As it is known, Tafel slope is determined by the rate-limiting step of HER41. The Tafel slope in our sample was measured to be about 92 mV/decade (Fig. 5b). Previous studies have shown a large range of Tafel slope from 40 mV to 140 mV/dec and the reaction mechanism on MoS2 still remains inconclusive18,19,20,21,42,43.
The charge-transfer resistance could be estimated by using electrochemical impedance spectroscopy (EIS) method, revealing a lower charge-transfer resistance than gold films (see Supplementary Fig. S8 online). The exchange current density, j0, is determined by fitting the linear portion of Tafel plot at low cathodic current to the Tafel equation (see Supplementary Fig. S9 online). Based on the results, it is found the exchange current density j0 is about 22.3 μA/cm2, which is 70 times of bulk MoS244. The large value of j0 is due to the high density of exposed edges20,21. However, the interlayer hopping of electrons between different MoS2 layers may limit the HER performance of vertically aligned MoS2 nanosheets42,45. Thus we believe the overall performance of our samples could be further improved by introducing doping during CVD process, which will be explored in the future. Another important criterion for a good electrocatalyst is its high durability. To evaluate this, continuous cyclic voltammograms were performed. The polarization curves before and after 1000 cycle are shown in Fig. 5c, where negligible loss of cathodic current could be observed.
In order to characterize the field-emission properties of vertically standing MoS2 nanosheets, a series of field emission measurement experiments were performed (Fig. 6). The anode was an indium tin oxide (ITO)-coated glass and the vertically standing MoS2 nanosheets grown on SiO2/Si substrate was used as the cathode. Figure 6a shows the field-emission current versus electric field of the as-prepared MoS2 nanosheets. The turn-on electric field (J = 10 μA/cm2) is around 2.46 V/μm, which is smaller than previous reported MoS2 nanosheets (~2.8–5.5 V/μm)25,46,47. Fowler-Nordheim (FN) theory is the most commonly used model for understanding the electron-emission behaviour of various nanostructures. A modified F-N equation is used here to analyze the field emission property of MoS2, which could be expressed as:
Field emission performance of vertically standing MoS2 nanosheets.
(a) Electron field emission current as a function of applied electric field. (b) The corresponding Fowler-Nordheim (ln(J/E2) vs 1/E) plot. (c) Field emission current stability curves of the MoS2 nanosheets. The inset shows the change of current density in detail. The sampling interval is 200 ms in both picture.

Where a = 1.54 × 10−6 A eV V−1 is a constant which depends on the surface structure and b = 6.83 × 107 V cm−1 eV−3/2, J is the emission current density, E is the applied average field, ϕ is the work function of emitter, λM is the macroscopic pre-exponential correction factor and vF (correction factor) is a particular value of the principal Schottky-Nordheim barrier function v48. The ratio of the actual local electric field and the applied average electric field is known as the field enhancement factor β. Fig. 6b shows the ln(J/E2) versus 1/E curve which has good agreement with the FN equation. The field-enhancement factor β of the vertical standing MoS2 sheets was calculated to be 6240 by taking the work function ϕ of bulk MoS2 to be 4.3 eV49. The large enhancement factor is due to the nanometric protrusions and sharp edges as we observed in Fig. 1.
The stability of emission current from the vertically standing MoS2 nanosheets was also measured (Fig. 6c). A stable emission current density of about 22 μA/cm2 over 170 min was recorded without any indication of degradation. Some spike like fluctuations were observed. The main reason of these fluctuations are believed to be caused by the adsorption/desorption and ion bombardment of residual molecules during the high field50. This test shows fairly stable emission current from vertically standing MoS2 nanosheets. The SEM observations on MoS2 nanosheets after field emission were carried out and shown in Supplementary Fig. S10. No severe deterioration of emitter surface was observed, demonstrating the stability of MoS2 nanosheets during field emission process.
Discussion
We have developed a CVD process for synthesizing vertically standing MoS2 nanosheets. High density MoS2 nanosheets with sharp edges could be synthesized. TEM observations on the nucleation sites reveal the growth mechanism for the based structure of vertically standing MoS2. The based structures act as the growth templet and promote the subsequently vertical growth of MoS2 nanosheets. It is suggested that the high strain energy caused by the compression between MoS2 islands are the main reason for vertically standing MoS2 nanosheets growth. These MoS2 nanosheets exhibit enhanced field-emission properties with low turn-on electric field and good emission stability, suggesting promising in field emission based devices applications. In addition, we further confirmed the catalytic activity in HER. A high exchange current density of ~28 μA/cm2 is achieved, which is caused by high density of the exposed edge sites. More generally, the ultrathin material, that is, the three-atoms-thick MoS2, together with its vertical morphology, would hold great promising potential in catalytic, sensor, field emission applications.
Methods
Growth method
The MoS2 growth was performed in a conventional quartz tube. Silicon substrates with 285 nm SiO2 layer were cleaned in Piranha solution, followed by acetone, isopropanol and deionized bath for 5 minutes and then finally dried using nitrogen gas. After that, samples were mounted on top of a quartz boat and faced down above high purity MoO3 powder (14 mg, 99.998%, Alfa Aesar). Also, 120 mg sulfur powder (99.5%, Alfa Aesar) was placed in a separate quartz boat located in the upstream of the quartz tube. The distance between sulfur powder and MoO3 powder was kept at 13cm. After that, the tube was pumped down to base pressure (<0.1Pa) and flushed with high purity nitrogen repeatedly. The tube was then filled with 1000 sccm nitrogen until one-atmosphere. During the synthesis process, the MoO3 was heated up to 750 °C at a rate of 15 °C/min in an argon environment at atmospheric pressure for 10 min. 5 sccm N2 was used as a carrying gas. Meanwhile, the sulfur was sublimated rapidly at approximately temperature of 700 °C. After growth procedure, the substrate was cooled down rapidly. Supplementary Fig. S11 shows the schematic diagram of the CVD equipment used in this study.
Characterization
The surface morphology which reveals the coverage and uniformity of the grown MoS2 nanosheets was observed directly by SEM (Quanta FEG 450). Raman spectroscopy (Horiba, LabRAM HR-800), atomic force microscopy (AFM, Vecco Nanoscope IIIa) and transmission electron microscopy (TEM, FEI Tecnai G2 F20) were used to further characterize structure and quality of the as-grown MoS2 nanosheets.
A diode setup in a vacuum chamber was adopted for field emission tests. The prepared samples were placed as the cathode and an indium tin oxide (ITO)-coated glass was used as the anode. Five 150-μm-thick and electrically insulating alumina films were used as spacers, making the distance between the cathode and anode at 250 μm (see Supplementary Fig. S12 online). The base pressure of the vacuum chamber was kept at 1 × 10−4 Pa. The emission current versus the applied voltage were characterized automatically by a Keithley 2410 sourcemeter and a high voltage DC power supply. The field emission current stability was investigated using a computer controlled data acquisition system.
In order to evaluate the catalytic effects of those vertically standing MoS2 nanosheets, the HER tests were carried out. All of the electrochemical measurements were performed in 0.5 M H2SO4 solution using a three-electrode steup on an electrochemical workstation, with a saturated calomel electrode as the reference electrode (SCE), MoS2 nanosheets grown on Au films the working electrode and a Pt foil the counter electrode. It was calibrated with respect to reversible hydrogen electrode (RHE). The calibration was performed in the high purity H2 saturated electrolyte with two Pt foils as the working electrode and counter electrode. In 0.5 M H2SO4, E (RHE) = E (SCE) + 0.252 V. All the potentials reported in our manuscript are against RHE. Linear sweep voltammetry was conducted with a scan rate of 5 mV/s. And AC impedance measurement was carried out at an overpotential (η) of 0.12V with an AC voltage of 5 mV.
Additional Information
How to cite this article: Li, H. et al. Synthesis and characterization of vertically standing MoS2 nanosheets. Sci. Rep. 6, 21171; doi: 10.1038/srep21171 (2016).
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Kuc, A., Zibouche, N. & Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfideTS2 . Phys. Rev. B 83 (2011).
Mak, K. F., Lee, C., Hone, J., Shan, J. & Heinz, T. F. Atomically ThinMoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 105 (2010).
Ramakrishna Matte, H. S. S. et al. MoS2 and WS2 Analogues of Graphene. Angew. Chem. 122, 4153–4156 (2010).
Osada, M. & Sasaki, T. Two-dimensional dielectric nanosheets: novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 24, 210–228 (2012).
Dean, C. R. et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 5, 722–726 (2010).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011).
Cui, X. et al. Multi-terminal transport measurements of MoS2 using a van der Waals heterostructure device platform. Nat. Nanotechnol. 10, 534–540 (2015).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2 . Nano Lett. 10, 1271–1275 (2010).
Liu, B. et al. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS nano 8, 5304–5314 (2014).
Putungan, D. B., Lin, S. H., Wei, C. M. & Kuo, J. L. Li adsorption, hydrogen storage and dissociation using monolayer MoS2: an ab initio random structure searching approach. Phys. Chem. Chem. Phys. 17, 11367–11374 (2015).
Acerce, M., Voiry, D. & Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 10, 313–318 (2015).
Romo-Herrera, J. M., Terrones, M., Terrones, H., Dag, S. & Meunier, V. Covalent 2D and 3D networks from 1D nanostructures: designing new materials. Nano Lett. 7, 570–576 (2007).
Wang, P. P., Sun, H., Ji, Y., Li, W. & Wang, X. Three-dimensional assembly of single-layered MoS2 . Adv. Mater. 26, 964–969 (2014).
Yu, J. H. et al. Vertical heterostructure of two-dimensional MoS(2) and WSe(2) with vertically aligned layers. Nano Lett. 15, 1031–1035 (2015).
Jiang, S. et al. Vertical ultrathin MoS2 nanosheets on a flexible substrate as an efficient counter electrode for dye-sensitized solar cells. Nanoscale 7, 10459–10464 (2015).
Xu, X., Faglioni, F. & Goddard, W. A. Methane activation by transition-metal oxides, MOx (M = Cr, Mo, W; x = 1, 2, 3). J. Phys. Chem. A 106, 7171–7176 (2002).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Li, Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Shi, J. et al. Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS Nano 8, 10196–10204 (2014).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Huang, C. K., Ou, Y. X., Bie, Y. Q., Zhao, Q. & Yu, D. P. Well-aligned graphene arrays for field emission displays. Appl. Phys. Lett. 98 (2011).
Huang, H. et al. Field electron emission of layered Bi2Se3 nanosheets with atom-thick sharp edges. Nanoscale 6, 8306–8310 (2014).
Jiang, L. et al. Controlled synthesis of large-scale, uniform, vertically standing graphene for high-performance field emitters. Adv. Mater. 25, 250–255 (2013).
Kashid, R. V. et al. Enhanced field-emission behavior of layered MoS2 sheets. Small 9, 2730–2734 (2013).
Hallam, T., Cole, M. T., Milne, W. I. & Duesberg, G. S. Field emission characteristics of contact printed graphene fins. Small 10, 95–99 (2014).
Kodambaka, S., Tersoff, J., Reuter, M. C. & Ross, F. M. Germanium nanowire growth below the eutectic temperature. Science 316, 729–732 (2007).
Wang, N., Cai, Y. & Zhang, R. Q. Growth of nanowires materials Science and Engineering R. Reports 60, 1–51 (2008).
Zhao, J., Shaygan, M., Eckert, J., Meyyappan, M. & Rummeli, M. H. A growth mechanism for free-standing vertical graphene. Nano Lett. 14, 3064–3071 (2014).
Li, H. et al. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 22, 1385–1390 (2012).
Late, D. J., Liu, B., Matte, H. S. S. R., Rao, C. N. R. & Dravid, V. P. Rapid Characterization of Ultrathin Layers of Chalcogenides on SiO2/Si Substrates. Adv. Funct. Mater. 22, 1894–1905 (2012).
Thripuranthaka, M., Kashid, R. V., Sekhar Rout, C. & Late, D. J. Temperature dependent Raman spectroscopy of chemically derived few layer MoS2 and WS2 nanosheets. Appl. Phys. Lett. 104, 081911 (2014).
Verble, J. L. & Wieting, T. J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 25, 362-& (1970).
Zhu, M. Y. et al. A mechanism for carbon nanosheet formation. Carbon 45, 2229–2234 (2007).
Shi, J. et al. Substrate Facet Effect on the Growth of Monolayer MoS2 on Au Foils. ACS nano 9, 4017–4025 (2015).
Yu, Y. et al. Controlled scalable synthesis of uniform, high-quality monolayer and few-layer MoS2 films. Scientific Reports 3, 1866 (2013).
Lin, Z. et al. Controllable Growth of Large-Size Crystalline MoS2 and Resist-Free Transfer Assisted with a Cu Thin Film. Scientific Reports 5, 18596 (2015).
V, Y., V, L. T., Boucaud, P., Debarre, D. & Bouchier, D. Kinetics of the heteroepitaxial growth of Ge on Si(001). J. Vac. Sci. Technol. B 20, 1251–1258 (2002).
Baskaran, A. & Smereka, P. Mechanisms of Stranski-Krastanov growth. J. Appl. Phys. 111, 044321 (2012).
Weiss, K. & Phillips, J. M. Calculated Specific Surface-Energy of Molybdenite (MoS2). Phys. Rev. B 14, 5392–5395 (1976).
Conway, B. E. & Tilak, B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2 and the role of chemisorbed H. Electrochim. Acta 47, 3571–3594 (2002).
Yu, Y. et al. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 14, 553–558 (2014).
Tao, L., Duan, X., Wang, C., Duan, X. & Wang, S. Plasma-engineered MoS2 thin-film as an efficient electrocatalyst for hydrogen evolution reaction. Chem Commun (Camb) 51, 7470–7473 (2015).
Seo, B. et al. Monolayer-Precision Synthesis of Molybdenum Sulfide Nanoparticles and Their Nanoscale Size Effects in the Hydrogen Evolution Reaction. ACS nano 9, 3728–3739 (2015).
Xie, J. et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 135, 17881–17888 (2013).
Li, Y. B., Bando, Y. & Golberg, D. MoS2 nanoflowers and their field-emission properties. Appl. Phys. Lett. 82, 1962 (2003).
Late, D. J. et al. Pulsed laser-deposited MoS2 thin films on W and Si: field emission and photoresponse studies. ACS Appl. Mater. Interfaces 6, 15881–15888 (2014).
Forbes, R. G. Extraction of emission parameters for large-area field emitters, using a technically complete Fowler-Nordheim-type equation. Nanotechnology 23, 095706 (2012).
Kim, S. et al. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 3, 1011 (2012).
Xiao, Z. et al. Field electron emission characteristics and physical mechanism of individual single-layer graphene. ACS Nano 4, 6332–6336 (2010).
Acknowledgements
The authors would thank the support from Tsinghua Nanofabrication Technology Center. This research was supported by the National Basic Research Program (2011CBA00600) and the National Natural Science Foundation (61474072). This work was also funded by Tsinghua National Laboratory for Information Science and Technology (TNList).
Author information
Authors and Affiliations
Contributions
H.L. and S.Y. synthesized and characterized the materials. H.L. conducted the electrochemical characterization and field emission experiments. H.L. and H.W. conducted the TEM measurements and analysis. H.L. and H.W. wrote and edited the manuscript. The project was supervised by H.W. and H.Q.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, H., Wu, H., Yuan, S. et al. Synthesis and characterization of vertically standing MoS2 nanosheets. Sci Rep 6, 21171 (2016). https://doi.org/10.1038/srep21171
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21171
This article is cited by
-
Single step growth of vertical MoS2 thin films by chemical vapor deposition for hydrogen evolution reaction
Journal of Materials Science: Materials in Electronics (2023)
-
Effect of the strain on spin-valley transport properties in MoS2 superlattice
Scientific Reports (2021)
-
Nonlinear Optical Properties of Vertically-Aligned MoS2 Nanosheets
Journal of Electronic Materials (2021)
-
Temperature dependant electronic charge transport characteristics at MX2 (M = Mo, W; X = S, Se)/Si heterojunction devices
Journal of Materials Science: Materials in Electronics (2019)
-
Evaluation of optical parameters and characterization of few layer sputtered MoS2 film by spectroscopic ellipsometry
Optical and Quantum Electronics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.