Abstract
The purpose of the present study was to investigate whether genetic variants that influence angiogenesis and sorafenib pharmacokinetics are associated with clinical outcomes and toxic effects in advanced renal cell carcinoma patients treated with this drug. One hundred patients with advanced renal cell carcinoma were enrolled. Forty-two polymorphisms in 15 genes were selected for genotyping and analyzed for associations with progression-free survival, overall survival and toxic effects. We found that rs1570360 in VEGF and rs2239702 in VEGFR2 were significantly associated with progression-free. Specifically, patients carrying the variant genotypes (AG + AA) of these two polymorphisms both had an unfavorable progression-free. In addition, compared with those with the rs2239702 GG genotype, patients with the AG + AA genotype suffered an unfavorable OS. We found that the VEGF rs2010963 CG + GG genotypes had a significantly increased risk of hand-foot syndrome and the ABCB1 rs1045642 CT + TT genotypes had an increased risk of high blood pressure. Our results suggest that polymorphisms in VEGF and VEGFR2 are associated with sorafenib clinical outcomes and polymorphisms in VEGF and ABCB1 are associated with sorafenib-related toxicities. Larger studies are warranted to validate our findings.
Similar content being viewed by others
Introduction
Renal cell cancer (RCC) is the predominant form of malignancy of the kidney (>80%) and accounts for about 3% of all adult malignancies and 2% of all cancer deaths1. Although the detection rate for RCC has increased due to improved diagnostic techniques, it is estimated that approximately 25% of patients already have metastatic disease at the time of diagnosis and 30% of surgically treated patients will develop metastases2,3. The outcome of patients with metastatic disease is dismal, as the 5-year survival rate is less than 10% and RCC is highly resistant to chemotherapy and radiotherapy. Cytokine therapy, such as high-dose interleukin-2, interferon-alpha, or both combined, has been used to treat advanced RCC4, but the low response rate and substantial side effects make this treatment only appropriate for a small number of selected cases.
Because angiogenesis has a pivotal role in tumor growth, strategies in cancer drug development have focused on inhibiting this pathway. Aberrant angiogenesis is considered a hallmark of RCC, particularly for clear cell RCC (ccRCC). In the majority of ccRCC cases, the von Hippel–Lindau tumor suppressor gene VHL is functionally disrupted, leading to constitutive activation of hypoxia-inducible factors (HIFs) and subsequent induction of target genes such as vascular endothelial growth factor (VEGF)5.
Sorafenib is a multi-targeting tyrosine kinase inhibitor against VEGF receptors, platelet-derived growth factor (PDGF) receptors, FMS-like tyrosine kinase 3 (FLT-3), rearranged during transfection (RET) gene, KIT and the RAF serine/threonine kinases6. Sorafenib was approved by the United States Food and Drug administration in December 2005 for treatment of metastatic RCC (mRCC) and is the first molecular-targeted drug used clinically for patients with advanced RCC in China. It seems to be more effective in patients of Chinese ethnicity than in western patients and is well tolerated with a manageable toxicity profile7.
In clinical practice, there is a wide range of clinical outcomes and degrees of toxic effects experienced by patients treated with sorafenib7. Therefore, the identification of biomarkers that could predict clinical response and toxic effects would aid in maximizing therapeutic efficacy and avoid unnecessary costs and side effects. In exploring individual susceptibility, some genetic variations such as polymorphisms within candidate genes have shown promising potential as biomarkers of clinical response and toxicity associated with tyrosine kinase inhibitor treatment8,9,10. A number of studies have proposed that polymorphisms affecting the pharmacokinetic and pharmacodynamic pathways of sunitinib and pazopanib (two drugs also used for mRCC) alter the efficacy of these drugs in the treatment of mRCC patients11,12,13. However, there is a paucity of studies investigating individual susceptibility to sorafenib treatment.
In the present study, we analyzed 42 polymorphisms within 15 genes known to be involved in the sorafenib pharmacokinetic and pharmacodynamic pathways and assessed the influence of these polymorphisms on clinical outcome and toxic effects in mRCC patients treated with sorafenib.
Methods and Patients
The Institutional Review Board of Nanjing Medical University, Nanjing, China approved this prospective study. At recruitment, all participants involved in this study provided written informed consent. The methods were carried out in accordance with the approved guidelines.
Between October 2006 and April 2012, 107 patients with diagnosed mRCC were recruited from the following institutions in China: First Affiliated Hospital of Nanjing Medical University (41 patients) and Jiangsu Cancer Hospital (9 patients) in Nanjing; Fudan University Shanghai Cancer Center (9 patients) and RENJI Hospital (10 patients) in Shanghai; Peking University First Hospital (7 patients) in Beijing; First People’s Hospital of Changzhou (9 patients) in Changzhou; Affiliated Hospital of Medical College Qingdao University (10 patients) in Qingdao; and Yangzhou First People’s Hospital (3 patients) in Yangzhou; Nantong Hospital of Traditional Chinese Medicine in Nantong (2 patients); Nanjing Jiangning Hospital in Nanjing (2 patients); Jiangyin People’s Hospital (3 patients) in Jiangyin and Wujiang NO.1 People’s Hospital (2 patients). Patients were excluded from participating if they had central nervous system metastasis, age outside of 18–80 y, Karnofsky performance status < 80%, life expectancy < 3 months, rheumatoid disease, or acute inflammation.
All the included patients had newly diagnosed mRCC without previous chemotherapy or radiotherapy. Their disease was classified in accordance with the criteria of the World Health Organization and the 2002 American Joint Committee on Cancer tumor-node-metastasis (TNM) classification. The pathology slides from radical nephrectomy or core biopsy were independently reviewed by two urological pathologists and were confirmed as ccRCC.
Sorafenib is the first molecular-targeted drug used for patients with mRCC in China. All the enrolled patients received sorafenib as their first-line treatment, which was given at 400 mg twice a day, orally, on a continual basis. Dosage modification to 800 mg twice a day was permitted if progression occurred and side effects could be tolerated. Treatment-related toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
The patients were followed-up every 2 months at the outpatient department from the time of enrollment. Seven patients withdrew from the treatment for economic reasons and were excluded from further analysis. Computed tomography scans were obtained at baseline and after every 2 cycles (12 weeks) of therapy (on average) according to the treating physician’s discretion. Objective response was recorded per investigator assessment according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The primary endpoints for this analysis of prognostic factors to sorafenib therapy were progression-free survival (PFS) and overall survival (OS).
Single nucleotide polymorphism (SNP) selection
We identified potentially functional polymorphisms in sorafenib pharmacokinetics genes (CYP3A4, CYP3A5, CYP1A1, CYP1A2, ABCB1 and ABCB2) or sorafenib pharmacodynamics genes (VEGF, VEGFR1, VEGFR2, VEGFR3, PDGFR, PDGFRB, IL8, HIF1A and EPAS1) according to the following criteria: (1) located in the 5′ flanking regions, 5′ untranslated region (UTR), 3′ UTR, or coding regions with amino acid changes; (2) minor allele frequency (MAF) > 5% in Chinese population. Besides, polymorphisms were reported to be significant in previous studies were also included in the present study. Finally, forty-two polymorphisms associated with sorafenib pharmacokinetics/pharmacodynamics were selected, as presented in online Table 1.
DNA extraction and genotyping
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Maxi kit (Qiagen, Valencia, CA, USA). Depending on the characteristics of the SNP, either SNaPshot or PCR-sequencing were applied for genotyping (Table 1). The primers and probes used for genotyping are available upon request.
Statistical analyses
The association between genotypes of SNPs and the rate of occurrence of side effects such as hand-food reaction, hypertension and diarrhea were assessed by chi-squared test and logistic regression analysis. PFS was defined as the time from first administration of sorafenib to the first documentation of disease progression or death from any cause. OS was considered the time between the first day of application of sorafenib and the date of death or last date of follow-up. PFS and OS were estimated by the Kaplan-Meier method and the log-rank test was used to compare different survival curves. Univariate or multivariate Cox regression analyses were performed to determine predictive factors of mRCC survival by estimating the crude hazard ratios (HRs), adjusted HRs and their 95% confidence intervals (CIs), with adjustment for possible confounders. All analyses were performed with the software SAS 9.1.3 (SAS Institute, Cary, NC, USA) with two-sided P-values. P < 0.05 was considered significant.
Results
Patient characteristics
The median age of the patients was 56 years (Table 2). Seventy-three of the 100 patients were men. Metastatic organs included lungs (69%), kidney (20%), bone (29%), lymph node (29%) and brain and skin (6%). Thirty-three percent of the patients had only one metastatic site.
Polymorphisms and OS of patients receiving sorafenib treatment
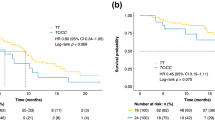
The median survival time (MST) of the cohort was 46.7 months (Fig. 1A). As shown in Table 3, we found that the SNP rs2239702, located in the 5′ UTR of VEGFR2, was significantly associated with unfavorable OS of the patients. Compared with the patients with the rs2239702 GG genotype, those with AG/AG had a shortened survival time (MST = 37.9 and 27.2, respectively, log-rank P = 0.041, Fig. 2).
Polymorphisms and PFS of patients receiving Sorafenib treatment
The MST of the patients was 31.8 months (Fig. 1B). Two polymorphisms, VEGFR2 rs2239702 (as aforementioned) and another polymorphism located in the 5′ UTR of VEGF (rs1570360) were significantly associated with the PFS of patients (Table 4). The MST of the patients with the VEGFR2 GG genotype was 43.2 months and for those with AG and AA genotypes were 22.5 and 25.2 months, respectively (log-rank P = 0.031). Furthermore, combined those with the variant genotypes (AG/GG), the patients with GG and AG/GG genotypes also had a significant difference in MST (MST = 43.2 and 24.5, respectively, log-rank P = 0.008, Fig. 3A,B). The VEGF rs1570360 was also associated with an inferior PFS. The MSTs for the patients with VEGF GG, AG, or AA, were 33.4, 20.3 and 4 months, respectively; and the difference was significant (log-rank P = 0.032). The difference in MST remained significant after combining the patients with variant genotypes AG/GG (MST = 33.4 and 18.2, for patients with GG and AG/AA genotypes, respectively; log-rank P = 0.034, Fig. 3C,D).
Polymorphisms and toxic effects of sorafenib
Table 4 presents the frequency of toxic side effects experienced during treatment by patients stratified by the analyzed polymorphisms, including hand-food reaction, hypertension and diarrhea. We found that there was a significant association between VEGF rs2010963 and the prevalence of hand-food reaction, which was more frequent in patients with the more unfavorable genotypes (rs2010963 CG and GG; P = 0.001, OR = 10.32, 95% CI = 2.10–68.63; online Table 5).
A polymorphism in ABCB1 (rs1045642) was found to be significantly associated with hypertension (Table 6) and patients with the more unfavorable genotypes (rs1045642 CT and CT/TT) experienced hand-food reaction more frequently (for TT cf. CC, P = 0.028, OR = 6.00, 95% CI = 1.22–29.53; for CT/TT cf. CC, P = 0.037, OR = 4.00, 95% CI = 1.09–14.67; Table 7)
Discussion
Research into molecular-targeted treatment has been the focus for mRCC in recent years, especially with regard to outcomes and toxicity. Differences have been noted in RCC sufferers receiving sunitinib or pazopanib that are associated with genetic polymorphisms11,12,13. Sorafenib, the first drug chosen to cope with mRCC in China, is currently being used in a number of patients. Considering the crucial role of angiogenesis and pharmacokinetic-related genes in influencing the efficacy of targeted therapy for RCC, we investigated associations between key SNPs in these genes and the clinical outcomes of patients treated with sorafenib. We found that polymorphisms in VEGF and VEGFR2 were associated with clinical outcomes of sorafenib treatment and polymorphisms in VEGF and ABCB1 were associated with sorafenib-related toxicities. To the best of our knowledge, this is the first study to explore the influence of genetic variants in angiogenesis and pharmacokinetic pathways on the clinical outcomes and toxic effects in mRCC patients treated with sorafenib. The study showed that genetic variations in VEGF and VEGFR2 were significantly associated with the PFS of RCC sufferers.
Carcinogenesis depends on the nutrition transported by blood vessels, the growth of which is determined by VEGF to a significant degree. For example, the inactivated VHL gene appears in nearly 60% of RCC patients. VHL protein, once inactivated, will decrease the number of degraded hypoxia-inducible factors [10878807]. Similarly, elevated levels of HIF-α can trigger the overexpression of VEGF, which then leads to stimulation of VEGFR and its downstream pathway, until tumor vessels generate14. Therefore, genetic variations in genes responsible for angiogenesis are crucial to the development of ccRCC, patients’ prognosis and the effect of drugs in targeted therapy. The crucial role of angiogenic signaling pathway in cancer has led to the development of medicines that inhibit VEGF receptor-targeted tyrosine kinase and have proven benefits in clinic use. The efficacy of these tyrosine kinase inhibitors in inhibiting several receptor tyrosine kinases in the angiogenic signaling pathway may be responsible for their efficacy.
In our previous studies, we found that VHL and HIF1A polymorphisms may contribute jointly to influence the progression and prognosis of RCC15. Garcia-Donas et al.16 found that VEGFR rs307826 and rs307821 as well as CYP3A5 rs776746 were significantly correlated with the PFS of RCC sufferers treated with sunitinib in a European population. As suggested by Kim et al.17, in metastatic ccRCC patients treated with sunitinib a combination of VEGF SNP 936 and VEGFR2 SNP 889 genotypes is associated with OS. In our study, we found that rs2239702, located in the 5′UTR of VEGFR2, was significantly associated with unfavorable OS of the patients. The median OS of rs2239702 GG carriers was significantly higher than that of GA or AA carriers.
In the analysis of PFS, two polymorphisms, VEGFR2 rs2239702 and another polymorphism located in the 5′UTR of VEGF (rs1570360), were significantly associated with PFS of the patients. Regarding VEGF rs1570360, the PFS of patients carrying the A allele was significantly shorter than the PFS of those carrying the G allele. The PFS of AA carriers was 33.4 months, AG 20.33 months and AA 4 months. For VEGFR2 rs2239702, it was found that the PFS of patients carrying the A allele was significantly shorter than for those carrying the G allele. These data suggest that alternative treatment approaches for patients with these genetic variants should be promoted.
Although the nature and incidence of adverse events related to sorafenib are currently well recognized and described, data regarding determinants of toxicity are still scarce. In our study, we found that there was a significant association between VEGF rs2010963 and the prevalence of hand-foot reaction. Although there has been little study addressing the role of the VEGF pathway in the pathophysiology of hand-foot reaction, Azad et al.18 provided evidence that inhibition of the VEGF pathway may be an important factor in sorafenib-related hand-foot reaction. Since rs2010963 is located in the 5′-UTR region of VEGF, it may alter VEGF expression by modulating promoter activity. Assuming that VEGF rs2010963 leads to decreased gene expression, it may mimic inhibition of VEGF and then confer susceptibility to hand-foot reaction. However, this deduction should be rigorously investigated in a future study.
In the present study, another polymorphism located in the exon region of ABCB1 (rs1045642) was found to be significantly associated with hypertension. ABCB1 is located in human chromosome 7 q21.1. ABCB1, a dominate gene in the human multidrug resistance gene family, can be regulated for its expression is decided by different factors12. Hoffmeyer et al.19 reported that the protein level of ABCB1 and the plasma drug levels of P-glycoprotein substrates were affected by polymorphisms in ABCB1. Their study revealed that P-glycoprotein levels in the duodenum of patients carrying 3435 CC were at least two-fold higher than that of patients carrying 3435 TT. The plasma drug levels dropped significantly when digoxin was given orally. These researches prove that high levels of P-glycoprotein affects drug absorption in the small intestine.
In 2007, Ebid et al.20 tested the plasma phenytoin level of epilepsy sufferers with ABCB1 C3435T. Polymorphisms in ABCB1 may result in individual differences in bioavailability and toxicity when drugs are taken orally, by changing drug absorption in the small intestine. Similar results have been found in research on sunitinib. Hand-foot reaction is associated with the copy sequence of haplotype TTT in ABCB1 (3435c/T, 1235 C/t, 2677 G/T), indicating that genetic variation in ABCB1 can affect toxicity and its severity when molecular-targeted drugs are administered11. Our present study showed that genetic variants that affect angiogenesis and pharmacokinetic pathways could also influence the occurrence of sorafenib-related toxicity in mRCC patients.
Our results herein suggest that polymorphisms in VEGF and VEGFR2 are associated with clinical outcomes of sorafenib treatment and polymorphisms in VEGF and ABCB1 are associated with sorafenib-related toxicities. As limitation of small sample size and lack of multiple comparison exit in the present study, the initial findings should be verified in the future studies. If confirmed, these genetic variants could provide the basis for individualized mRCC treatment.
Additional Information
How to cite this article: Qin, C. et al. The influence of genetic variants of sorafenib on clinical outcomes and toxic effects in patients with advanced renal cell carcinoma. Sci. Rep. 6, 20089; doi: 10.1038/srep20089 (2016).
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics. CA Cancer J Clin 63, 11–30 (2013).
Cohen, H. T. & McGovern, F. J. Renal-cell carcinoma. N Engl J Med 353, 2477–90 (2005).
Drucker, B. J. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev 31, 536–45 (2005).
Law, T. M. et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 76, 824–32 (1995).
Baldewijns, M. M. et al. VHL and HIF signalling in renal cell carcinogenesis. J Pathol 221, 125–38 (2010).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356, 125–34 (2007).
Ye, D. W. & Zhang, H. L. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. Onco Targets Ther 7, 925–35 (2014).
Vaziri, S. A. et al. Vascular endothelial growth factor polymorphisms: role in response and toxicity of tyrosine kinase inhibitors. Curr Oncol Rep 12, 102–8 (2010).
Schneider, B. P., Radovich, M. & Miller, K. D. The role of vascular endothelial growth factor genetic variability in cancer. Clin Cancer Res 15, 5297–302 (2009).
Erdem, L. et al. Polymorphisms to predict outcome to the tyrosine kinase inhibitors gefitinib, erlotinib, sorafenib and sunitinib. Curr Top Med Chem 12, 1649–59 (2012).
van, Erp. N. P. et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol 27, 4406–12 (2009).
van, der. Veldt. A. A. et al. Genetic polymorphisms associated with a prolonged progression-free survival in patients with metastatic renal cell cancer treated with sunitinib. Clin Cancer Res 17, 620–9 (2011).
Xu, C. F. et al. Pazopanib efficacy in renal cell carcinoma: evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol 29, 2557–64 (2011).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3, 721–32 (2003).
Qin, C. et al. The polymorphisms in the VHL and HIF1A genes are associated with the prognosis but not the development of renal cell carcinoma. Ann Oncol 23, 981–9 (2012).
Garcia-Donas, J. et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 12, 1143–50 (2011).
Kim, J. J. et al. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer 118, 1946–54 (2012).
Azad, N. S. et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res 15, 1411–6 (2009).
Hoffmeyer, S. et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 97, 3473–8 (2000).
Ebid, A. H., Ahmed, M. M. & &Mohammed, S. A. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit 29, 305–12 (2007).
Acknowledgements
Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Program for Development of Innovative Research Team in the First Affiliated Hospital of Nanjing Medical University, Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, National Natural Science Foundation of China (81171963, 81201571, 81372757 and 81402321) and Jiangsu Provincial Special Program of Medical Science (BL2012027).
Author information
Authors and Affiliations
Contributions
S.P., J.X., Y.C. and Z.Z. managed the project. W.J., Z.L., L.X., Y.D., Z.H., H.Y., D.B., S.X., Z.Q., C.H., S.L., Z.J., L.F., J.J., C.L., W.X., Z.H., Z.H., W.B., C.J. and J.M. collected and prepared samples. Q.C., C.Q. and L.P. wrote the paper. W.S., W.M., C.H. and C.Q. performed the statistics analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Qin, C., Cao, Q., Li, P. et al. The influence of genetic variants of sorafenib on clinical outcomes and toxic effects in patients with advanced renal cell carcinoma. Sci Rep 6, 20089 (2016). https://doi.org/10.1038/srep20089
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20089
This article is cited by
-
PIK3R5 genetic predictors of hypertension induced by VEGF-pathway inhibitors
The Pharmacogenomics Journal (2022)
-
Clinical Pharmacokinetics and Pharmacodynamics of Transarterial Chemoembolization and Targeted Therapies in Hepatocellular Carcinoma
Clinical Pharmacokinetics (2019)
-
Impact of CYP3A4*22 on Pazopanib Pharmacokinetics in Cancer Patients
Clinical Pharmacokinetics (2019)
-
Genetic polymorphisms associated with adverse reactions of molecular-targeted therapies in renal cell carcinoma
Medical Oncology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.