Abstract
The role of thyroid transcription factor 1 (TTF-1) in the diagnosis of metastatic pulmonary adenocarcinomas in pleural, pericardial and peritoneal effusions has not been defined. This study aimed to assess the overall diagnostic accuracy of TTF-1 for metastatic pulmonary adenocarcinomas in pleural or other effusions. Literature search was conducted in PubMed, EMBASE and other databases to find eligible publications. Quality was assessed according to standardized QUADAS-2 criteria. Sensitivity, specificity, positive/negative likelihood ratio (PLR/NLR) and diagnostic odds ratio (DOR) were pooled. Summary receiver operating characteristic (SROC) curves were used to assess overall performance of the TTF-1 assay. A systematic search revealed 20 studies comprising a total of 1,213 subjects in this meta-analysis. The summary estimates were listed as follows: sensitivity, 0.74 (95% CI: 0.69–0.79); specificity, 0.99 (95% CI: 0.97–1.00); PLR, 78.16 (95% CI: 27.15–225.05); NLR, 0.26 (95% CI: 0.22–0.32); and diagnostic odds ratio, 297.75 (95% CI: 104.16–851.19). Estimated positive and negative post-probability values for metastatic pulmonary adenocarcinomas prevalence of 20% were 95% and 6%, respectively. The area under the SROC curve was 0.96. TTF-1 shows significant potential as a diagnostic marker to differentiate metastatic pulmonary from non-pulmonary adenocarcinomas in pleural or other effusions. These results justify larger, more rigorous studies to confirm such a diagnostic role.
Similar content being viewed by others
Introduction
Adenocarcinoma has become the most common histological type of lung cancer, approximately half of lung cancer patients are diagnosed with adenocarcinoma and the incidence is rapidly increasing worldwide1,2. Metastatic pulmonary adenocarcinomas often manifest as pleural effusion, while some cases present as pericardial, or peritoneal effusions3. It is important to identify the primary site of adenocarcinomas in pleural, pericardial and peritoneal effusions, since this information guides disease treatment and management, as well as prognosis assessment4. However, identifying adenocarcinoma origin can be challenging; for example, it is difficult or even impossible to differentiate metastatic pulmonary adenocarcinomas from non-pulmonary adenocarcinomas on the basis of morphology and effusion samples5. Positive cytology examination may suggest the presence of malignant diseases, but it may not indicate the primary site of adenocarcinoma.
Immunostaining can help identify the site of origin, but most adenocarcinoma markers are not organ-specific. This highlights the need for more biologically specific markers for pulmonary adenocarcinomas in order to distinguish metastatic pulmonary from non-pulmonary adenocarcinomas in pleural, pericardial and peritoneal effusions6,7. Thyroid transcription factor 1 (TTF-1) is a homeodomain-containing transcription factor selectively expressed in the thyroid, diencephalon and lung8. TTF-1 was recently proposed as an immunohistochemical marker of pulmonary adenocarcinomas, with one meta-analysis reporting overall sensitivity of 76% and specificity of 100% in tissue samples9. TTF-1 also plays a role in the diagnosis of malignant effusion10. Studies suggest that TTF-1 is a potential biomarker for differentiating pulmonary adenocarcinomas from non-pulmonary adenocarcinomas in pleural or other effusions11,12,13, but results from these studies have not always been consistent. Therefore we meta-analyzed the available evidence on whether TTF-1 can distinguish metastatic pulmonary adenocarcinomas from non-pulmonary adenocarcinomas in pleural or other effusions.
Material and Methods
This meta-analysis was conducted and reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews, the Meta-analysis Statement and methods recommended by the Cochrane Diagnostic Test Accuracy Working Group14,15. There was no need for institutional review board approval for this retrospective meta-analysis.
Literature search
Two investigators (Y. Shen and C. Pang) searched in PubMed, EMBASE, Web of Knowledge, CNKI, WANFANG and WEIPU databases for relevant articles published up to May 2015. The following search terms were used as Medical Headings and/or text words: “Thyroid transcription factor 1 OR TTF-1” AND “pleural effusion OR pleural fluid OR hydrothorax OR ascites OR peritoneal effusion OR pericardial effusion OR serous effusion” AND “sensitivity OR specificity OR accuracy”. Reference lists of the included studies and review articles were also checked to identify additional studies.
Selection of eligible studies
A study was included if it fulfilled the following criteria: (i) it examined the ability of TTF-1 to differentiate metastatic pulmonary adenocarcinomas from non-pulmonary adenocarcinomas in humans; (ii) it analyzed pleural, pericardial and peritoneal effusions as samples; (iii) it reported sufficient data to allow calculation of true positive (TP), false positive (FP), false negative (FN) and true negative (TN) rates; (iv) it reported definitive determination of metastatic pulmonary adenocarcinomas and non-pulmonary adenocarcinomas using gold-standard methods; and (v) it was an original research study published in English or Chinese. Conference proceedings and studies published only as abstracts were excluded. To avoid selection bias, we also excluded studies involving fewer than 20 patients. When several articles concerned the same subjects, only results from the publication with the largest sample were used.
Data extraction
Two reviewers (Y. Shen and C. Pang) independently identified eligible studies and extracted data on study methodology, characteristics and test accuracy using a standardized extraction form. The data extracted were: name of first author, publication year, country, serous effusion types, sample preparation method, TTF-1 immunostaining method, antibody clone and dilution and two-by-two tables of TP, TN, FP and FN. Detailed information about controls with non-pulmonary adenocarcinoma was also reviewed.
Assessment of methodological quality
The same two reviewers (Y. Shen and C. Pang) assessed the quality of the selected studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria, which cover four key domains for assessing risk of bias and applicability of the study results. These domains are patients selection, index test, reference standard and flow and timing of samples/patients through the study16. Any discrepancies between the two authors (Y. Shen and C. Pang) during study selection, data extraction or quality assessment were resolved by discussion with a third author (K. Shen).
Statistical analysis
We used standard methods recommended for bivariate meta-analysis of diagnostic test evaluations17. We descriptively analyzed study characteristics and QUADAS-2 quality assessment using Excel and Review Manager 5.2 (The Cochrane Collaboration, Copenhagen, Denmark). The following measures of test accuracy were computed for each study, together with 95% confidence intervals (95% CIs): sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR). A summary ROC (SROC) curve covering all the studies was plotted using the data on sensitivity and specificity for a single test threshold from each study. The area under the SROC curve (AUC) was used to summarize the overall diagnostic performance of TTF-1.
The heterogeneity effect was measured using the Q test and the inconsistency index (I2). P < 0.05 or I2 ≥ 50% indicated significant heterogeneity, which was then analyzed through meta-regression to identify potential covariates. Deeks’s funnel plot was used to detect publication bias18. Post-test probability was calculated using the overall prevalence of 20% with Fagan nomograms. All analyses were performed using the “Midas” module in STATA 12.0 (Stata Corp., College Station, TX) and Meta-DiSc 1.4 for Windows (XI, Cochrane Colloquium, Barcelona, Spain). All statistical tests were two-sided, with P < 0.05 taken as the threshold for statistical significance.
Results
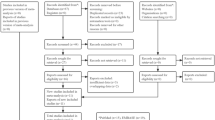
Systematically searching literature databases and manually searching reference lists in relevant reviews and studies identified 20 studies examining the diagnostic accuracy of TTF-1 in pleural or other effusions in patients with metastatic pulmonary adenocarcinomas19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38. Studies were excluded because they were not diagnostic studies, they did not report sufficient data to construct 2 × 2 tables, or they mixed other type of cancers like squamous-cell carcinoma. The process of selecting of studies eligible for inclusion is shown in Fig. 1.
Patient characteristics and study design
The final set of 20 studies involved 1,213 subjects, comprising 668 patients with metastatic pulmonary adenocarcinomas and 545 controls with non-pulmonary adenocarcinomas (median 60 patients per study; range 32–113 patients). (Table 1). Thirteen studies were performed in Asia, six in the USA and one in Europe. The most frequent cancer types among the 545 patients with non-pulmonary adenocarcinomas were breast (n = 178), gastrointestinal (n = 147) and ovary adenocarcinomas (n = 145). Eight studies assayed pleural effusion; four studies, pleural effusion, pericardial effusion and ascites; another four studies, pleural effusion and ascites; two studies, only mentioned as serous effusions; one study, pleural effusion and pericardial effusion; and one study, only pericardial effusion. Only two studies involved analysis of smear samples25,34; the remainder relied on analysis of cell blocks. Table 2 summarizes individual study designs and results for diagnostic performance of TTF-1.
Most studies detected TTF-1 using the 8G7G3/1 antibody; three used the SPT24 antibody33,34,37 and one study did not report this information35. Twelve studies used immunohistochemistry to detect TTF-1, while the remaining eight used immunocytochemistry. Antibody dilutions from 1:40 to 1:500 were used in the included studies, while five did not report dilution factors. All studies defined nuclear staining as positive. Supplemental Table 1 summarizes the clinical information of patients with non-pulmonary adenocarcinomas.
Methodological quality of the included studies
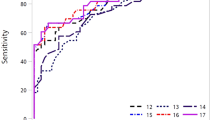
QUADAS-2 was proposed in 2011 as an improved redesign of the original QUADAS and it was integrated into RevMan 5.2 in 2012. We applied the four criteria of QUADAS-2 (patient selection, index test, reference standard, flow and timing) to the studies in our meta-analysis. A response of “Yes” was given if the criterion was fulfilled, “Unclear” if fulfillment was unclear and “No” if the criterion was not fulfilled. Based on these responses, the risk of bias for each criterion was classified as low, high, or unclear. Based on the first three domains, the applicability of the results was also evaluated. The quality of included studies was generally good, but three studies19,21,27 were at high risk of bias due to deficiencies in patient selection. Figure 2 shows the summary of QUADAS-2 assessments of included studies.
Summary of QUADAS-2 assessments of included studies.
QUADAS-2: Quality Assessment of Diagnostic Accuracy Studies-2. Patient Selection: Describe methods of patient selection; Index Text: Describe the index test and how it was conducted and interpreted; Reference Standard: Describe the reference standard and how it was conducted and interpreted; Flow and Timing: Describe any patients who did not receive the index tests or reference standard or who were excluded from the 2 × 2 table and describe the interval and any interventions between index tests and the reference standard. (From Ann Intern Med. 2011; 155(8):529–36.)
Diagnostic accuracy of TTF-1
Sensitivity of TTF-1 for diagnosing metastatic pulmonary adenocarcinomas in effusions was between 0.54 and 0.88 and the pooled sensitivity was 0.74 (95% CI: 0.69–0.79). Specificities of TTF-1 varied from 0.92 to 1.00 and the pooled specificity was 0.99 (95% CI: 0.97–1.00). The other pooled parameters for TTF-1, calculated over all 20 studies, were: PLR, 78.16 (95% CI: 27.15–225.05); NLR, 0.26 (95% CI: 0.22–0.32); and DOR, 297.75 (95% CI: 104.16–851.19) (Fig. 3).
Figure 4 shows a plot of the TP rate as a function of the FP rate in individual studies, as well as the corresponding SROC curve. The AUC was 0.96, indicating a high discriminatory ability for TTF-1. Fagan’s nomogram for likelihood ratios (Fig. 5) indicated that using TTF-1 to detect metastatic pulmonary adenocarcinomas increased the post-probability to 95% when the results were positive and reduced the post-probability to 6% when the results were negative.
Summary receiver operating characteristic (SROC) curves for the detection of metastatic pulmonary adenocarcinoma using thyroid transcription factor-1.
The SROC curve with confidence and prediction regions around mean operating sensitivity and specificity point analyses of TTF-1. AUC, area under the curve; SENS, sensitivity; SPEC, specificity.
Meta-regression and publication bias
I2 values for diagnostic performance indices were as follows: sensitivity, 51.7% (P = 0.00); specificity 30.2% (P = 0.10); PLR 0.00% (P = 0.16); NLR, 57.57% (P = 0.00); and DOR, 99.20% (P = 0.00). This suggests high heterogeneity among included studies, so, a meta-regression was performed to identify possible sources of heterogeneity. The meta-regression featured six covariates: (i) country of origin (Asia vs. non-Asia); (ii) TTF-1 assay method (immunohistochemistry vs. immunocytochemistry), (iii) TTF-1 clone (8G7G3/1 vs. other); (iv) TTF-1 antibody dilution (≤1:150 vs. >1:150 and other), (v) study design (prospective vs. retrospective); and (vi) blinding (blind vs. other). None of these covariates was found to be a significant source of heterogeneity (all P > 0.05, Table 3).
Deeks’s funnel plot asymmetry test was used to assess likelihood of publication bias in the included 20 studies. The slope coefficient was associated with P = 0.11, suggesting symmetry in the data and low likelihood of such bias (Fig. 6).
Discussion
Diagnosing lung adenocarcinoma based on resection histology is normally straightforward, but diagnosing metastatic pulmonary adenocarcinomas based on effusion samples can be extremely challenging5,6. Available biomarkers in effusions differentiate poorly between pulmonary adenocarcinomas and non-pulmonary adenocarcinomas. TTF-1 has emerged as a promising candidate biomarker, but studies of its diagnostic performance have given conflicting results11,12,13. Our meta-analysis is strengthened by the use of a standard protocol, strict inclusion criteria, standardized data extraction, independent reviewers and a bivariate random-effects model39. Our meta-analysis of available evidence suggests that TTF-1 can accurately predict whether an adenocarcinoma cell originated from a pulmonary or non-pulmonary site. However, TTF-1 probably cannot stand on its own and so should be used in conjunction with other markers.
Our meta-analysis indicated that TTF-1 performed with medium sensitivity (0.74, 95% CI: 0.69–0.79); and high specificity (0.99, 95% CI: 0.98–1.00), with a relatively high rate of missed diagnoses (16%) but a low rate of misdiagnosis (1%). These findings suggest that TTF-1 is a highly specific marker of pulmonary adenocarcinoma origin in pleural and other effusions. The SROC curve, which assesses overall test performance by showing the trade-off between sensitivity and specificity40, had an AUC of 0.96, suggesting high overall accuracy. Another indicator of diagnostic accuracy is DOR, which combines sensitivity and specificity data into a single number ranging from 0 to infinity, with higher values indicating better discriminatory test performance. Mean DOR in our meta-analysis was 297.75, suggesting that assaying TTF-1 should be helpful in the diagnosis of metastatic pulmonary adenocarcinomas. We further examined the diagnostic accuracy of TTF-1 by calculating PLR and NLR, which can be easier to relate to clinical practice than SROC and DOR. The pooled PLR value of 78.16 suggests that patients with metastatic pulmonary adenocarcinomas have an approximately 78-fold higher chance of giving a positive TTF-1 result than do patients without metastatic pulmonary adenocarcinomas. At the same time, the pooled NLR was 0.26, indicating that a negative TTF-1 result is still 26% likely to be a false negative, which is not low enough to rule out metastatic pulmonary adenocarcinomas.
The relatively low sensitivity of TTF-1 in identifying metastatic pulmonary adenocarcinoma cells in effusion samples means that it is probably not sufficiently reliable on its own. Instead it should be used in conjunction with other markers. For example, combining of TTF-1 and napsin A gave higher sensitivity and accuracy than TTF-1 alone in identifying metastatic pulmonary adenocarcinomas34. Carcino-embryonic antigen is often targeted during immunostaining of metastatic pulmonary adenocarcinoma in pleural or other effusions41, so, including TTF-1 within a panel of immunostaining markers such as CEA and napsin A, may increase the overall sensitivity and specificity, thereby improving overall accuracy.
Though TTF-1 may play a role in identifying malignant effusions, comparing the diagnostic performance of TTF-1 with that of classical tumor markers such as CA15-3 and vascular endothelial growth factor (VEGF) is difficult, because the two types of biomarker serve different purposes. Immunostaining for TTF-1 is done primarily to determine the source of malignant cells. When measured by ELISA on pleural fluid supernatants, TTF-1 had a poor diagnostic accuracy for differentiating malignant and benign effusions with the sensitivity of only 9%10. Examining markers such as CA 15-3 and VEGF, or using other diagnostic tools such as percutaneous pleural biopsy and VATS-directed biopsy, is done to determine whether effusions are malignant or benign42,43,44,45.
TTF-1 is also a sensitive marker for papillary carcinoma of the thyroid, although it is estimated that fewer than 1% of patients with papillary thyroid carcinoma have malignant pleural effusions46. In this meta-analysis, only one case of thyroid carcinoma was reported in 545 patients with non-pulmonary adenocarcinomas. The rarity of metastatic thyroid carcinoma in serous effusions explains the nearly 100% specificity of TTF-1 in detecting metastatic lung adenocarcinoma across several studies.
Our meta-analysis results indicated an association between TTF-1 and presence of metastatic pulmonary adenocarcinomas, implying that TTF-1 may contribute to such metastasis. Winslow et al. reported that downregulation of TTF-1 is associated with loss of differentiation, enhanced tumor seeding ability and increased metastatic potential in lung adenocarcinoma47. Positive and partially positive TTF-1 expression in lung adenocarcinoma patients correlates with EGFR mutations (exon 19 and 21). In clinical practice, the combination of TTF-1 expression and EGFR mutations, especially mutations in exon 21, can guide timely clinical treatment for lung adenocarcinomas48. Future studies should examine how TTF-1 functions in lung adenocarcinoma-related regulatory and signaling pathways. At the same time, researchers and clinicians should not overextend their interpretations of TTF-1 expression, which should be taken into account only when malignant cells are present. Indeed, the requirement for malignant cells limits the diagnostic sensitivity and clinical significance of TTF-1 and distinguishes it from assays based on circulating tumor DNA or classical tumor markers.
Standardized techniques for detecting TTF-1 should be established in order to maximize the clinical utility of this biomarker. Studies should rigorously determine whether immunohistochemistry or immunocytochemistry is superior and the dilution of primary antibody should be optimized. Dilution factors among the studies in this meta-analysis ranged from 1:40 to 1:500. Studies should also compare the different primary antibodies available; one study has suggested that the SPT24 antibody clone is better than the 8G7G3/1 clone49. It may also be possible to improve sensitivity or specificity of immunohistochemical staining by optimizing antibody cut-off values50.
The findings of this meta-analysis should be interpreted with caution because of several limitations. While our strict inclusion and exclusion criteria may have helped reduce selection bias, they led to a relatively small final set of studies for which statistical power may be inadequate for drawing definitive conclusions about the ability of TTF-1 to discriminate metastatic pulmonary adenocarcinomas from metastatic non-pulmonary adenocarcinomas in pleural or other effusions. For example, we included only studies published in English and Chinese in a relatively small number of databases. Our results may be biased by our omission of unpublished studies, studies published in other languages and studies published in journals not indexed in the databases we searched. In addition, we detected substantial heterogeneity across the included studies, for which we were unable to identify causes using meta-regression. Future studies should aim for greater rigor in order to decrease the risk of bias.
Conclusions
In summary, our meta-analysis suggests that TTF-1 may significantly aid the diagnosis of metastatic pulmonary adenocarcinomas in pleural or other effusions. Our data provide further evidence that TTF-1 is a useful marker for distinguishing metastatic adenocarcinoma of the lung from non-pulmonary adenocarcinoma in specimens of pleural or other effusions.
Additional Information
How to cite this article: Shen, Y. et al. Diagnostic value of thyroid transcription factor-1 for pleural or other serous metastases of pulmonary adenocarcinoma: a meta-analysis. Sci. Rep. 6, 19785; doi: 10.1038/srep19785 (2016).
References
Houston, K. A., Henley, S. J., Li, J., White, M. C. & Richards, T. B. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer 86, 22–8 (2014).
Zugazagoitia, J., Enguita, A. B., Nuñez, J. A., Iglesias, L. & Ponce, S. The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: current concepts and future prospects. J. Thorac Dis 6, S526–36 (2014).
Wu, S. G. et al. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 41, 1409–18 (2013).
Azzopardi, M., Porcel, J. M., Koegelenberg, C. F., Lee, Y. C. & Fysh, E. T. Current controversies in the management of malignant pleural effusions. Semin Respir Crit Care Med 35, 723–31 (2014).
Pereira, T. C., Saad, R. S., Liu, Y. & Silverman, J. F. The diagnosis of malignancy in effusion cytology: a pattern recognition approach. Adv Anat Pathol 13, 174–84 (2006).
Chowdhuri, S. R., Fetsch, P., Squires, J., Kohn, E. & Filie, A. C. Adenocarcinoma cells in effusion cytology as a diagnostic pitfall with potential impact on clinical management: a case report with brief review of immunomarkers. Diagn Cytopathol 42, 253–8 (2014).
Fetsch, P. A. & Abati, A. Immunocytochemistry in effusion cytology: a contemporary review. Cancer 93, 293–308 (2001).
Ordóñez, N. G. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 20, 429–44 (2012).
Li, L. et al. The high diagnostic accuracy of combined test of thyroid transcription factor 1 and Napsin A to distinguish between lung adenocarcinoma and squamous cell carcinoma: a meta-analysis. PLoS One 9, e100837 (2014).
Porcel, J. M. et al. TTF-1 and napsin A on cell blocks and supernatants of pleural fluids for labeling malignant effusions. Respirology 20, 831–3 (2015).
Li, X. et al. Thyroid transcription factor-1 amplification and expressions in lung adenocarcinoma tissues and pleural effusions predict patient survival and prognosis. J. Thorac Oncol 7, 76–84 (2012).
Pomjanski, N. et al. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagn Cytopathol 33, 309–15 (2005).
Dinu, M., Ciurea, R. N., Stefan. M. & Georgescu, A. C. The role of immunohistochemistry in the diagnosis of neoplastic pleural effusions. Rom J. Morphol Embryol 53, 817–20 (2012).
Moher, D., Liberati, A., Tetzlaff, J., Altman. D. G. & PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097 (2009).
Leeflang, M.,. M.,, Deeks, J. J., Takwoingi. Y. & Macaskill, P. Cochrane diagnostic test accuracy reviews. Syst Rev 2, 82 (2013).
Whiting, P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155, 529–36 (2011).
Shen, Y. et al. CT-base pulmonary artery measurement in the detection of pulmonary hypertension: a meta-analysis and systematic review. Medicine (Baltimore) 93, e256 (2014).
Deeks, J. J., Macaskill, P. & Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58, 882–93 (2005).
Hecht, J. L., Pinkus, J. L., Weinstein, L. J. & Pinkus, G. S. The value of thyroid transcription factor-1 in cytologic preparations as a marker for metastatic adenocarcinoma of lung origin. Am J Clin Pathol 116, 483–8 (2001).
Jang, K. Y., Kang, M. J., Lee, D. G. & Chung, M. J. Utility of thyroid transcription factor-1 and cytokeratin 7 and 20 immunostaining in the identification of origin in malignant effusions. Anal Quant Cytol Histol 23, 400–4 (2001).
Afify, A. M. & al-Khafaji, B. M. Diagnostic utility of thyroid transcription factor-1 expression in adenocarcinomas presenting in serous fluids. Acta Cyto l46, 675–8 (2002).
Gomez-Fernandez, C., Jorda, M., Delgado, P. I. & Ganjei-Azar, P. Thyroid transcription factor 1: a marker for lung adenoarinoma in body cavity fluids. Cancer 96, 289–93 (2002).
Ng, W. K., Chow, J. C. & Ng, P. K. Thyroid transcription factor-1 is highly sensitive and specific in differentiating metastatic pulmonary from extrapulmonary adenocarcinoma in effusion fluid cytology specimens. Cancer 96, 43–8 (2002).
Su, X., Jiang, L., Li, G., Xie, B. & Jiang, Y. Expression of thyroid transcription factor-1 on metastatic pulmonary adenocarcinoma cells in serous effusions (Chinese). J. Clin Exp Pathol 20, 706–708 (2004).
Jan, I. S. et al. Utility of thyroid transcription factor-1 expression in the differential diagnosis of metastatic adenocarcinoma of serous effusion specimens prepared using the cell transfer technique. J. Formos Med Assoc 105, 695–700 (2006).
Dejmek, A. et al. Napsin A (TA02) is a useful alternative to thyroid transcription factor-1 (TTF-1) for the identification of pulmonary adenocarcinoma cells in pleural effusions. Diagn Cytopathol 35, 493–7 (2007).
Zhu, W. & Michael, C. W. WT1, monoclonal CEA, TTF1 and CA125 antibodies in the differential diagnosis of lung, breast and ovarian adenocarcinomas in serous effusions. Diagn Cytopathol 35, 370–5 (2007).
Zhou, C., Shen, H. & Li, X. Expression and gene mutation analysis of thyroid transcription factor-1 in metastatic adenocarcinoma cells of pleural effusion (Chinese). J Clin Exp Pathol 23, 685–689 (2007).
Jiang, B., Wu, G. P., Zhao, Y. J. & Wang, S. C. Transcription expression and clinical significance of TTF-1 mRNA in pleural effusion of patients with lung cancer. Diagn Cytopathol 36, 849–54 (2008).
Wang, J. & Xu, M. Clinical pathogenic significance of examination of Napsin A, SP-A and TTF-1 in pleural effusion (Chinese). Tianjin Yi Yao 36, 967–968 (2008).
Kim, J. H. et al. Utility of thyroid transcription factor-1 and CDX-2 in determining the primary site of metastatic adenocarcinomas in serous effusions. Acta Cytol 54, 277–82 (2010).
Khoor, A., Byrd-Gloster, A. L. & Nicosia, S. V. Expression of thyroid transcription factor-1 in malignant pleural effusions. Pathol Oncol Res 17, 263–7 (2011).
Kim, J. H., Kim, Y. S., Choi, Y. D., Lee, J. S. & Park, C. S. Utility of napsin A and thyroid transcription factor 1 in differentiating metastatic pulmonary from non-pulmonary adenocarcinoma in pleural effusion. Acta Cytol 55, 266–70 (2011).
Liu, L., Cohen, C. & Siddiqui, M. T. Thyroid transcription factor 1 and napsin a double staining in lung adenocarcinoma in pleural fluid. Acta Cytol 56, 425–30 (2012).
Liu, Y., Liu, L. & Lv, X. Expression of TTF-1 on pulmonary adenocarcinoma cells in serous effusions (Chinese). Hebei Med J 34, 1658–59 (2012).
Luo, Q., Jiang, H. & Zhang, J. The value of immunocytochemistry in the distinguishment of primary site of metastatic adenocarcinoma in effusions (Chinese). Xian Dai Zhong Liu Yi Xue 20, 822–24 (2012).
Yan, J. et al. A fine decision tree consisted of CK5/6, IMP3 and TTF1 for cytological diagnosis among reactive mesothelial cells, metastatic adenocarcinoma of lung and non-lung origin in pleural effusion. Int J Clin Exp Pathol 7, 5810–8 (2014).
Yin, J. et al. Expression of Napsin A, TTF-1 and CK-7 in pericardial effusion due to metastatic lung adenocarcinoma (Chinese). Can Res Clin 26, 119–121 (2014).
Reitsma, J. B. et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58, 982–90 (2005).
Hamza, T. H., van, Houwelingen, H. C., Heijenbrok-Kal, M. H. & Stijnen, T. Associating explanatory variables with summary receiver operating characteristic curves in diagnostic meta-analysis. J Clin Epidemiol 62, 1284–91 (2009).
Li, Q., Bavikatty, N. & Michael, C. W. The role of immunohistochemistry in distinguishing squamous cell carcinoma from mesothelioma and adenocarcinoma in pleural effusion. Semin Diagn Pathol 23, 15–9 (2006).
Nguyen, A. H., Miller, E. J., Wichman, C. S., Berim, I. G. & Agrawal, D. K. Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res 166, 432–9 (2015).
Shen. Y. C. et al. Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: A meta-analysis. Exp Ther Med 3, 1072–1076 (2012).
Chakrabarti, B., Ryland, I., Sheard, J., Warburton, C. J. & Earis, J. E. The role of Abrams percutaneous pleural biopsy in the investigation of exudative pleural effusions. Chest 129, 1549–55 (2006).
Sayir, F., Cobanoglu, U., Mergan, D. & Demir, H. Video-assisted thoracoscopic surgery for malignant pleural effusions. Asian Pac J Cancer Prev 12, 415–8 (2011).
Vassilopoulou-Sellin, R. & Sneige, N. Pleural effusion in patients with differentiated papillary thyroid cancer. South Med J 87, 1111–6 (1994).
Winslow, M. M. et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 473, 101–4 (2011).
Shanzhi, W., Yiping, H., Ling, H., Jianming, Z. & Qiang, L. The relationship between TTF-1 expression and EGFR mutations in lung adenocarcinomas. PLoS One 9, e95479 (2014).
La. Rosa. S. et al. TTF1 expression in normal lung neuroendocrine cells and related tumors: immunohistochemical study comparing two different monoclonal antibodies. Virchows Arch 457, 497–507 (2010).
Smits, A. J., Vink, A., Tolenaars, G., Herder, G. J. & Kummer, J. A. Different Cutoff Values for Thyroid Transcription Factor-1 Antibodies in the Diagnosis of Lung Adenocarcinoma. Appl Immunohistochem Mol Morphol 23, 416–21 (2015).
Acknowledgements
National Natural Science Foundation of China (81230001 to Fuqiang Wen and 81300032 to Yongchun Shen); Projects in the Science and Technology Pillar Program from the Department of Science and Technology of Sichuan Province (2015SZ0151 to Yongchun Shen). We are indebted to the authors of the primary studies included in this meta-analysis; without their contributions, this work would not have been possible.
Author information
Authors and Affiliations
Contributions
Conception and design: Y.S., C.P. and F.W. Development of methodology: K.S., Y.W., C.W. and D.L. Acquisition of data: Y.S., C.P. and K.S. Analysis and interpretation of data: Y.S., C.P., Z.L., T.Y. and L.C. Writing, review and/or revision of the manuscript: Y.S., C.P. and K.S. Administrative, technical, or material support: K.S., Y.W. and C.W., Z.L. Study supervision: F.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shen, Y., Pang, C., Shen, K. et al. Diagnostic value of thyroid transcription factor-1 for pleural or other serous metastases of pulmonary adenocarcinoma: a meta-analysis. Sci Rep 6, 19785 (2016). https://doi.org/10.1038/srep19785
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19785
This article is cited by
-
The role of IgE specific for galactose-α-1,3-galactose in predicting cetuximab induced hypersensitivity reaction: a systematic review and a diagnostic meta-analysis
Scientific Reports (2020)
-
Wisteria floribunda agglutinin-positive Mac-2-binding protein as a diagnostic biomarker in liver cirrhosis: an updated meta-analysis
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.