Abstract
The mammalian cochlea is a highly specialized organ within the inner ear. Sensory hair cells (HC) in the cochlea detect and transduce sound waves into electrical impulses that are sent to the brain. Studies of the molecular pathways regulating HC formation are hindered by the very sparse nature of HCs, where only ~3300 are found within an entire mouse cochlea. Current cell lines mimic certain aspects of HCs but lack terminal HC marker expression. Here we successfully “pseudo-immortalized” cochlear progenitor cells using the “conditional reprogramming” technique. These cells, termed “Conditionally Reprogrammed Otic Stem Cells” (CR-OSC), are able to bypass the senescence inherent to cochlear progenitor cells without genetic alterations, allowing for the generation of over 15 million cells from a single cochlea. These cells can be differentiated and up-regulate both early and terminal differentiation genes associated with HCs, including the terminal HC differentiation marker prestin. CR-OSCs also respond to known HC cues, including upregulation of HC genes in response to Atoh1 overexpression and upregulation of prestin expression after thyroid hormone application. Overall, we describe the creation of a HC line capable of regulated expression of HC genes that can easily be recreated in any laboratory from any mouse of interest.
Similar content being viewed by others
Introduction
Auditory hair cells (HCs) are mechanosensory cells in the cochlea that are critical for audition. HCs are highly specialized cells that are present in relatively low abundance with approximately 3300 HCs per mouse cochlea1. Two types of HCs exist within the cochlea, the inner hair cells, which are primarily responsible for the detection and transduction of sound into neuronal signaling and the outer hair cells (OHCs), which are electromotile and act as a cochlear amplifier2,3,4. Electromotility of OHCs is controlled by the non-traditional motor protein prestin5, which is coded for by the Slc26a5 gene and is a unique protein expressed in OHCs. Without the amplification provided by prestin/OHCs, mice suffer a substantial loss of hearing3,4 demonstrating the importance of this protein for auditory function.
Despite the crucial role for prestin in the cochlea, relatively little is known about the transcriptional regulation of prestin. Thyroid hormone (TH) was the first factor discovered to regulate prestin expression based on observations that hypothyroidism can result in hearing abnormalities6,7,8. It was later demonstrated that TH binds directly to and activates prestin9,10,11. Later studies correlated transcription factors such as Pou4f3 with prestin expression11, but these studies have been unable to further clarify the mechanisms underlying these correlations. One of the major limiting factors for the study of prestin regulation is the lack of an appropriate system to analyze. Most studies to date have been performed in cochlear explants, vastly limiting the material available, the speed at which experiments can be done and dramatically increasing the cost of the experiment. Indeed, this is true for investigations into the regulation of any genes or proteins expressed specifically in HCs.
To bridge this gap, multiple cell lines have been developed to aid in the study of HC development or to be used as screening tools for the prevention of ototoxicity. Many of these cell lines were created from the “immorto-mouse”12,13,14 and exhibit several aspects of HCs15,16. These cell lines have been used to identify dozens of compounds and pathways that ameliorate ototoxic effects of cisplatin or aminoglycoside antibiotic treatment17,18,19. Although these cell lines have proven useful for ototoxic screening studies, they have not been ideal for studying terminal HC differentiation. Additionally, studies have shown that some of these cell lines have begun to show significant phenotypic drift and are no longer sensitive to aminoglycoside induced cell death20,21.
Lineage restricted auditory progenitor cells, often called otic spheres, or otic stem cells, can be isolated from embryonic and postnatal cochleae22,23,24 and differentiated into cells which bear many hallmarks of a HC22,23,24,25, including the ability to express the terminal HC gene, prestin, under differentiating conditions25. While promising, these cells can only be grown for a few generations, yielding only thousands of cells25, limiting their use for large scale studies. In other epithelial tissues, such as breast and prostate, the passage number limitations inherent to lineage restricted progenitor cells can be overcome by “conditional reprogramming” (CR) of the cells26,27,28. This procedure involves growing lineage restricted progenitor cells on a layer of feeder cells, while treating the cells with an inhibitor of rho associated kinases (ROCK). Amazingly, this procedure allowed for the unrestricted growth of the breast and prostate progenitor cells, without compromising their ability to differentiate into mature breast or prostate tissues when put into differentiation conditions26. Since the organ of Corti is also an epithelial tissue, we asked whether the CR procedure could be applied to otic progenitor cells and if the CR-otic progenitor cells could prove useful for the study of terminal hair cell genes, such as prestin.
In this study, we exposed otic progenitor cells to CR conditions (termed Conditionally Reprogrammed Otic Stem Cells, CR-OSCs) and found that they respond similarly to CR-breast and prostate tissue. Primarily, CR-OSCs can be grown for many generations and can easily generate 15 million cells from 10 primary otic spheres. Similar to breast and prostate cells, CR-OSCs demonstrated an increase in the expression of telomerase over passage number. Remarkably, once we removed these cells from CR conditions and transferred them into differentiation conditions, the CR-OSCs transcriptionally upregulated nearly every tested gene for hair cell maturation, including the terminal maturation marker prestin. Finally, we tested whether CR-OSCs could regulate transcription of hair cell genes in response to two known pro-HC manipulations: ectopic expression of Atoh1, an early pro-HC transcription factor and application of TH which promotes prestin transcription. After ectopic Atoh1 expression, early HC markers were upregulated, mirroring what has been observed in previous studies29,30,31,32,33,34. Consistent with known effects of TH on HCs in cochlear explants11, application of TH (either T3 or T4) to CR-OSCs resulted in a dramatic upregulation of prestin expression. Combined, these data demonstrate that CR-OSCs can respond to pro-HC manipulations via the upregulation of HC-specific transcripts.
In total we have described the creation of a novel, easy-to-generate cell line capable of expressing many genes characteristic of differentiated hair cells including the terminal differentiation gene prestin. The ease of this procedure allows any laboratory to quickly create CR-OSCs from any mouse model, including genetically modified mouse lines, or crosses of genetically modified mouse lines. Thus, a large number of CR-OSCs can be obtained from Cre/LoxP, rtTA/tTA, or fluorescent reporter mice. These can then be used to expedite fate-mapping and knock-out and knock-in studies, as well as the development of reporter and mutant hair cell lines to study the regulation of HC specific genes. Additionally, creating cell lines from genetic mouse models in this way should provide for less cell-to-cell variability than viral transduction studies. Though, our work here shows that CR-OSCs can also be virally transduced allowing many ways for the researcher to ask questions about gene function in otic progenitor cells. Finally, CR-OSCs can produce vast quantities of cells making large scale downstream applications feasible; from next-generation sequencing to high throughput screening. CR-OSCs will provide hearing researchers a powerful new tool to investigate the genetic regulation of HC maturation and potentially regeneration.
Methods
Animals
The mouse strains used in this study include: prestin-CreER35, CAG-Cre36, Ai14-tdTomato36 and prestin-YFP (Yamashita et al., in press). All animal work conducted during the course of this study was approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital and was performed according to NIH guidelines.
Harvesting Otic Spheres
Whole organs of Corti were separated from the stria vascularis and from the modiolus by micro-dissection from postnatal day (P) 2-4 mice, into Hank’s Balanced Salt Solution with no calcium and no magnesium (Life Technologies) and digested with 0.125% trypsin-EDTA for 10 minutes. Enzymatic digestion was halted with the addition of 10 mg/mL soybean trypsin inhibitor (Life Technologies) and 1 mg/mL DNase I (Sigma Aldrich). Cells were triturated gently 20 times using a 1000 μL pipette tip to be single cell suspension and passed through a 40-μm cell strainer. The cells were grown in renewal media consisting of: DMEM/F12 (Life Technologies) with supplement of 1× N2 (Life Technologies), 1 × B27 (Life Technologies), 50 ng/mL Ampicillin (Fisher Scientific), mouse epidermal growth factor (20 ng/ml), mouse basic fibroblast growth factor (10 ng/ml), insulin-like growth factor-1 (50 ng/mL; all growth factors purchased from R&D Systems) and heparin sulfate (50 ng/mL; Sigma). After 1–3 days, solid spheres were identified and harvested.
Conditional Reprogramming of Otic Spheres
Solid otic spheres were placed into a 6-well plate coated in a layer of mitomycin c (Enzo Life Sciences) inactivated (10 μg/mL, 3 hours) 3T3-J2 cells (Swiss 3T3 fibroblasts J2 strain, less than passage 20, 6 × 105/well, gift from Dr. Schlegel26), NIH/3T3 cells (ATCC CRL-1658), or primary MEFs in CR media at 5% CO2 and 37 °C. CR media consisted of F medium (3:1 (v/v) F-12 Nutrient Mixture: (Ham)–Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen), 5% fetal bovine serum (FBS, Life Technologies), 0.4 μg/mL hydrocortisone (Sigma-Aldrich), 5 μg/mL insulin (Sigma-Aldrich), 8.4 ng/mL cholera toxin (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Invitrogen) and 24 μg/mL adenine (Sigma-Aldrich) with addition of 5 μM Y-27632 (ROCK inhibitor, Enzo Life Sciences)26 for 7 days (medium change every 2–3 days) when large mitotically active colonies of CR-OSCs were identified and harvested manually and placed into differentiation conditions22,25: poly-L-ornithine and fibronectin treated 6-well plates, in media containing: DMEM/F12 (Life Technologies) supplemented with 1x N2 (Life Technologies), 1x B27 (Life Tech), 50 ng/ml Ampicillin (Fisher Scientific) for 14 days (1 medium change after 7 days). For passage of CR-OSCs, mitotically active colonies were identified and exposed to TrypLE (Life Technologies) for 15 min at room temperature continuously monitored under a microscope. After 15 min the CR-OSC colonies were preferentially digested and carefully removed from the feeder cells. Any colonies that were not removed by 15 min were manually scraped from the surface and harvested. CR-OSCs were spun down at 500 g for 10 min and re-suspended in fresh CR media. 50,000 cells were re-plated onto a 6-well plate coated with feeder cells to start the next passage.
Cell Culture
Human embryonic kidney cells (HEK) were obtained from ATCC (CRL-1573, less than 20 Passages); NIH/3T3 cells (ATCC CRL-1658) were a generous gift from Dr. Suzanne Baker (St. Jude Children’s Research Hospital); and the primary mouse embryonic fibroblasts (MEFs) were gifted by Dr. Guillermo Oliver (St. Jude Children’s Research Hospital). These cells were maintained at 37 °C with 5% CO2 in DMEM (Life Technologies) supplemented with 10% FBS (Life Technologies) and 1x penicillin/streptomycin (Life Technologies). HEI-OC1 cell line (A gift from Dr. Kalenic and the House Research Institute14) was grown under permissive conditions: 10% CO2, 33 °C and DMEM + 10% FBS + 50 ng/mL ampicillin (Life Technologies).
Viral transduction
Adeno-Associated Virus (AAV) subtypes 2/5 overexpressing Atoh1 or GFP (Vector Bioloabs) were added to a final concentration of 2.5 × 1011 genome copies/mL into a 96-well plate containing 1–2 large CR-OSC colonies (approximately 5,000 cells) or 5,000–10,000 HEK or 5,000 HEI-OC1 cells for either 2 or 7 days after which the mRNA was harvested and analyzed.
Quantitative Real Time PCR
Total RNA was harvested using RNA-Stat 60 (Tel-Test Inc.) and 200 ng of total RNA was converted to cDNA using High-Capacity cDNA Reverse Transcription Kit (Life Technologies), then diluted to 1 ng/μL cDNA in ddH20. 2 ng were used for multiplexed qPCR using Taqman Mastermix (Life Technologies) following the manufacturer’s instructions. qPCR was performed using a Mastercycler Realplex2 (Eppendorf) real time PCR machine.
qPCR Primers
Primer/probes were obtained from Life Technologies FAM: Atoh1 (Mm00476035_s1), Pou4f3 (Mm04213795_s1), myosin VI (Mm00500651_m1), myosin VIIa (Mm01274015_m1), parvalbumin (Mm00443100_m1), otoferlin (Mm00453306_m1), prestin (Mm00446145_m1), VGlut3 (Mm00805413_m1), telomerase (Mm00484957_m1). VIC: 18 s (4319413E).
Immunohistochemistry
Differentiated CR-OSCs were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 15 minutes. Immunostaining was performed with the myosin VI rabbit polyclonal antibody (1:200, Proteus BioSciences) followed by Tyramide Signal Amplification (TSA) kit (Life Tech) following the manufacturer’s instructions, or with GFP chicken polyclonal antibody (1:500, Abcam) followed by fluorescein-conjugated secondary antibody. Nuclei were visualized by a 20 minute incubation with Hoechst33342 (1:2000, Life Tech) in PBS at room temperature. Cells were imaged using an Operetta High Content Imaging System (Perkin Elmer) or a Zeiss LSM 780 inverted confocal microscope (Zeiss).
Statistics
Statistics were performed using Origin 8.5 (OriginLab). Samples were compared using a standard two sample T test and where multiple comparisons were performed the p-values were adjusted using the Bonferroni correction.
Results
Lineage Restricted Otic Progenitor Cells can be Pseudo-immortalized
To test if CR conditions developed for breast/prostate spheres could be successfully applied to the organ of Corti, we isolated otic-spheres from postnatal day P2-4 wild-type (wt) mouse cochleae and exposed them to the CR conditions. After 7 days, large colonies were observed growing in the mitotically inactive feeder cells (Fig. 1A, white arrows, colonies circled by white dashed lines) and eventually displaced the feeder cells (Fig. 1B, dead feeder cells indicated by red arrow heads). To ensure these colonies were from our mice and not feeder cell contamination, we performed the same procedure using Rosa26-CAG-TdTomato mice36, where all cells within the mice express the red fluorescent protein tdTomato. After 7 days, large, red colonies were seen growing in and actively displacing the feeder cells which did not exhibit any red fluorescence (Fig. 1C). To test the requirement of Y-27632 or the feeder cells, we attempted to reprogram otic spheres without either the Y-27632 or without the feeder cells and observed no reprogramming in the absence of either of these factors, indicating that both ROCK inhibition and the presence of the feeder cells are necessary for the process (data not shown).
Lineage Restricted Otic-Stem cells can be conditionally reprogrammed.
(A) P2-P4 otic-spheres were placed into conditional reprogramming conditions and grown for 7 days. Large growing colonies (white arrows and circled by white dashed lines) were observed in the mitotically inactive feeder cells. (B) After 14 days feeder cells are actively displaced from the plate (red arrow heads) as the growing colonies overtake the plate. (C) Otic-spheres were harvested from Rosa26-CAG-TdTomate mice and placed into conditional reprogramming conditions for 7 days, after which they were stained with cell mask blue and imaged. Large TdTomato positive colonies were observed growing in the feeder cells when examined under fluorescent microscopy. (D,E) Otic-spheres grew on NIH3T3 cells (D) or primary MEFs (E) for 14 days.
To extend the general applicability, we used MitoC-treated NIH3T3 cells or primary MEFs as the feeders. Interestingly, we found that although otic-spheres could grow on both NIH3T3 and MEFs, the growing speed was slower (Fig. 1D,E) and no colonies were observed after second passage. These results suggest J2 cells are the most suitable CR feeder cells.
Characterization of Conditionally Reprogrammed Otic Stem Cells (CR-OSCs)
To better characterize the CR-OSC cells, we initially monitored their growth over 10 generations and asked how their growth rate changed with each passage. CR-OSCs were maintained in 1 well of a 6-well plate and continually passaged for 10 passages. Every 7 days CR-OSCs were harvested, counted and 50,000 CR-OSCs were re-plated for the next passage. CR-OSCs generated over 15 million cells throughout the 10 passages studied (Fig. 2A), a vast improvement to the tens of thousands of cells which can be produced from otic spheres prior to the onset of senescence25. Furthermore, the growth rate did not appear to change over the 10 passages (Fig. 2B); averaging approximately 1.6 million cells generated every passage (Fig. 2B, dashed line). Previous work done on breast and prostate spheres demonstrated a robust increase in telomerase expression during the CR passages. Similarly, CR-OSCs demonstrated an increase in the transcription of telomerase for several passages (Fig. 2C, 1° (primary passage)-2° (secondary passage) p = 0.005, 1°–3° p = 0.003, 1°–4° p = 0.028 1°–5° p = 7 × 10−4). Finally, we sought to differentiate between two different hypotheses that could explain the increased proliferation observed in the CR-OSCs. It is possible that the prolonged proliferation seen in CR-OSCs was due to the CR conditions recruiting normally non-dividing cells to proliferate, or alternatively the existing pool of dividing cells could have been coaxed into continued proliferation. To discriminate between the two, we trypsinized primary otic spheres into a single cell suspension and plated half of the suspension into CR conditions and half to form new secondary otic spheres. We reasoned if equal numbers of new secondary spheres formed as did CR-OSC colonies it would eliminate the possibility that normally non-dividing cells were recruited when the cells are in CR conditions. After 7 days we counted the new secondary spheres and compared it against CR-OSC colonies. We observed no significant difference between the two (Fig. 2D, n = 15, p = 0.75), implying that normally non-dividing cells are not the source of the increased proliferation seen in CR-OSCs.
Characterization of Conditionally Reprogrammed Otic Stem Cells (CR-OSCs).
(A) 50,000 CR-OSC cells were plated starting at passage 2 and grown for 7 days. After 7 days the cells were differentially trypsinized from the remaining feeder cells, counted and 50,000 of them were re-plated for the next passage. The cumulative total of cells produced during this period was plotted against passage number (n = 3, slope = 1.534 × 106, R2 = 0.992). (B) The number of cells produced after 7 days in culture was plotted against passage number (n = 3). Dotted blue line represents the overall average across all generations, 1.61 × 106. (C) The relative expression of telomerase normalized to the 18s ribosomal subunit was compared for the first 5 passages of CR-OSCs against passage 1 (n = 10). (D) Otic-spheres were isolated and trypsinized into a single cell suspension. Half of the suspension was plated into CR conditions and the other half plated to form new secondary spheres. After 7 days the number of new CR colonies or secondary spheres were counted and plotted (n = 15). *indicates p < 0.05 corrected by Bonferroni method. Mean ± S.E.M.
CR-OSCs Transcriptionally Up-regulate Hair Cell Genes in Response to Differentiation Conditions
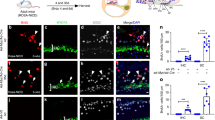
To characterize how CR-OSCs regulate known HC genes in response to a differentiation cue, we manually harvested and plated primary CR-OSC colonies into differentiating conditions. After 14 days we performed immunohistochemistry against a common, highly expressed hair cell marker, myosin VI and observed 5.97 ± 0.96% myosin VI positive cells in differentiated CR-OSCs (Fig. 3A). To examine if CR-OSCs also express prestin, a terminal outer hair cell differentiation marker, we cultured otic spheres from prestin-YFP mice, in which the endogenous prestin is tagged with YFP. Interestingly, we found less than 1% CR-OSCs expressed YFP signal after 21 days (Fig. 3B). Double labeling myosin VI and prestin-YFP showed all YFP positive cells are myosin VI positive (Fig. 3C, white arrow heads), indicating that a portion of myosin VI-positive cells terminally differentiated and expressed prestin.
CR-OSCs Transcriptionally Upregulate Hair Cell-specific Genes in Response to Differentiation Conditions.
(A) Primary passage CR-OSC colonies were manually harvested and plated into differentiation conditions. After 14 days the cells were fixed, stained for myosin VI (red) or Hoechst (blue) and imaged. (B) Primary passage CR-OSC colonies from Prestin-YFP mice were manually harvested and plated into differentiation conditions for 21 days after which the cells were fixed and immune-labeled by YFP antibody. (C) Primary passage CR-OSCs were differentiated for 21 days and stained for myosin VI (red), YFP (green) and Hoechst (blue). (D) Primary passage CR-OCS colonies were harvested before (0 Days) and after (14 Days) differentiation. The expression of transcripts were normalized to 18s RNA. N = 15 for all conditions. *indicates p < 0.05. Mean ± S.E.M.
To better characterize the regulation of HC markers, we performed quantitative real time PCR (qPCR) against a panel of eight HC markers spanning early to terminal maturation states (Atoh1, Pou4f3, myosin VI, myosin VIIa, parvalbumin, otoferlin, VGlut3 and prestin) and compared expression of these markers between 0 and 14 days of differentiation conditions. Under both situations all eight genes were expressed at detectable levels (Fig. 3D), but Atoh1 (p = 0.014), Pou4f3 (p = 0.019), myosin VI (Fig. 3F, p = 0.046), myosin VIIa (p = 0.025), parvalbumin (p = 0.006) and prestin (p = 0.013) were significantly upregulated under differentiating conditions for 14 days.
CR-OSCs Transcriptionally Upregulate Hair Cell Genes in Response to Differentiation Condition Regardless of Passage Number
We have demonstrated that CR-OSCs can be passaged repeatedly. Here, we sought to characterize the expression levels of HC genes against the passage number. We passaged CR-OSCs through 5 passages, manually harvested and differentiated CR-OSC colonies after each passage, followed by qPCR. The first 4 passages had no change in the expression levels of any tested gene (Fig. 4A–H). However, after the fifth passage, myosin VI (Fig. 4C, p = 0.029) and myosin VIIa (Fig. 4D, p = 0.013) were expressed at levels significantly lower than their maxima (passage 1 for myosin VI and passage 2 for myosin VIIa), though still significantly higher than undifferentiated CR-OSCs (red dashed line, myosin VI 6.75 fold increase over undifferentiated, myosin VIIa 14.5 fold increase over undifferentiated). All other HC genes (Atoh1, Pou4f3, Parvalbumin, otoferlin, prestin, VGlut3) demonstrated no change in expression levels after any passage assayed by quantitative real time PCR.
The Effect of Passage Number on the Ability of CR-OSCs to Transcriptionally Upregulate Hair Cell Genes in Response to Differentiation Conditions.
(A–H) CR-OSC colonies were continually passaged for 5 generations and CR-OSCs were manually harvested after each passage and plated into differentiation conditions for 14 days, after which the colonies were harvested and the relative expression (normalized to 18s) was calculated for: Atoh1 (A), Pou4f3 (B), myosin VI (C) myosin VIIa (D) parvalbumin (E), otoferlin (F), VGlut3 (G), or prestin (H) and plotted relative to expression levels of the primary passage. N = 15 for all conditions. *indicates p < 0.05 corrected by Bonferroni. Mean ± S.E.M.
CR-OSCs Upregulate Pou4f3, Myosin VI and Otoferlin in Response to Atoh1 Overexpression
We next tested if CR-OSCs responded to a common pro-HC cue, overexpression of Atoh1, which has been demonstrated to upregulate HC-specific genes in non-HCs and promote an immature HC fate in vivo29,31,37. We transduced AAV containing CAG driven Atoh1 into CR-OSCs, as well as an AAV containing a CAG driven GFP as a negative control. We also transduced HEK cells and HEI-OC1 cells, a hair cell line commonly used in testing small molecule compounds for otoprotection, to compare how different cell lines performed in response to Atoh1 overexpression. We chose to use the permissive conditions for the HEI-OC1 cells, as these conditions are the mostly commonly used for screening, express several HC markers14 and have a substantially lower rate of cell death14. AAV-Atoh1 was transduced during differentiation of CR-OSCs for either 2 days or 7 days, or for only 2 days in HEK and HEI-OC1 cells due to their proliferative nature. After transduction with AAV-Atoh1, colonies were harvested and assayed by qPCR. Atoh1 levels were significantly higher in all cases (Fig. 5A, CR-OSC 2DIV p = 0.009; CR-OSC 7DIV p = 0.05; HEK 2DIV p = 2.9 × 10−4; HEI-OC1 p = 6.76 × 10−6) but only CR-OSCs had upregulation of HC genes in response to Atoh1 overexpression (Fig. 5B: Pou4f3 2DIV, p = 0.009; Pou4f3, 7DIV p = 0.024; Fig. 5C myosin VI, 7DIV p = 0.035; Fig. 5C otoferlin, 7DIV, p = 0.031). Notably, overexpressing Atoh1 failed to induce prestin, consistent with previous studies that demonstrated that overexpression of Atoh1 was not sufficient to force terminal maturation of HCs29,31,37. Thus, our results indicate CR-OSCs respond to Atoh1 overexpression similar to otic progenitor cells under in-vivo or ex-vivo conditions, while HEK and HEI-OC1 cells have almost no response.
CR-OSCs Upregulate Pou4f3, Myosin VI and Otoferlin in Response to Atoh1 Overexpression.
(A–H) Primary CR-OSC colonies were manually harvested and plated into differentiation conditions for 14 days. After 7, or 12 days (corresponding to 7 days or 2 days of viral transduction) 1.0 × 1012 genome copies/mL of AAV-Atoh1 or AAV-GFP virus was added to the media. Similarly HEK or HEI-OC1 cells were plated and 1.0 × 1012 genome copies/mL of virus was added for 2 days. Following treatment, cells were harvested and expression of Atoh1 (A), Pou4f3 (B), myosin VI (C) myosin VIIa (D) parvalbumin (E), otoferlin (F), VGlut3 (G), or prestin (h) was determined, normalized to 18s. Data is plotted against the control GFP viral transductions. N = 10 for all conditions. *indicates p < 0.05 corrected by Bonferroni method. Mean ± S.E.M.
CR-OSCs Upregulate Prestin in Response to Thyroid Hormone Treatment
Finally, we asked whether CR-OSCs can up-regulate prestin expression in response to known mediators of prestin mRNA expression. We tested the responsiveness of CR-OSCs to the prohormone Thyroxin (T4) and the 4-fold more potent form, Triiodothyrone (T3), which has been described to potentiate prestin expression in OHCs11. Quantitative PCR revealed that application of either T3 (Fig. 6A, p = 0.032) or T4 (Fig. 6B, p = 0.042) caused a significant upregulation of prestin mRNA in CR-OSCs, while no detectable level of prestin was observed in either HEK or HEI-OC1 cells following similar treatments. These results again demonstrate the similarities between the CR-OSCs and cochlear OHCs in the upregulation of prestin expression by thyroid hormones.
CR-OSCs Upregulate Prestin in Response to Thyroid Hormone Treatment.
(A,B) Primary CR-OSC colonies were manually harvested and placed into differentiation conditions for 14 days. HEK cells or HEI-OC1 cells were also tested. Triiodothyronine (A) or Thyroxine (B) were added to the culture media for 2 days at either 400 nM (red) or 200 μM (blue). N = 10 for all conditions. *indicates p < 0.05 corrected by Bonferroni method. Mean ± S.E.M.
Discussion
In this study we explored the conditional reprogramming technique, which was designed to allow the unlimited proliferation of breast and prostate progenitor cells without affecting their lineage restricted differentiation potential26. Since breast and prostate cells as well as the cells that comprise the organ of Corti are all epithelial, we hypothesized that the conditional reprogramming technique would allow for the continued proliferation of otic progenitor cells. We found that the conditional reprogramming technique was able to increase the number of cells generated from otic progenitor cells from the 104 cells currently reported25 to 107 CR-OSCs. Additionally, we did not observe any senescence before the experiments were arbitrarily ended at 10 passages. We were even able to passage one line for 25 passages. These data suggest that if one desired greater numbers of CR-OSCs, the procedure could easily be scaled up by seeding with more than 50,000 cells, or by continuing to plate cells beyond the 10th passage. Additionally, CR-OSCs appear to recapitulate the ability of CR treated breast and prostate progenitor cells to differentiate into their lineage-restricted cell types when placed into differentiation conditions. CR-OSCs actively up-regulated numerous HC genes including early differentiation genes like Atoh1 and Pou4f3 as well as terminal differentiation genes such as prestin in response to differentiation conditions.
CR-OSCs offer many advantages over traditional methodologies: first and foremost, the ability to generate millions of cells from a single cochlea; cells that allow for monitoring and manipulation of the expression of many HC specific genes which should allow researchers to save time, resources and animals when asking questions pertaining to transcriptional regulation of HC-specific genes. Second, the simplicity of this procedure allows for any laboratory to generate CR-OSCs. The conditional reprogramming procedure involves only 2 steps: 1) plating otic progenitors onto a layer of mitotically inactivated 3T3 feeder cells; 2) addition of a Rho associated kinase (ROCK) antagonist to the F-media. Third, this procedure is transient and does not involve the manipulation of a cell’s genome to achieve the increased growth. The transient nature and ease of the procedure allows CR-OSC to be generated from a primary culture of otic progenitor cells and allows one to easily establish and re-establish CR-OSC cell lines from live mice to minimize genetic drift.
Surprisingly, our stock of HEI-OC1 cells completely lacked prestin expression even though HEI-OC1 cells were previously shown to express prestin by immunohistochemistry in both the permissive conditions (the conditions used in this study) and non-permissive conditions14. This observation was also evident in the Thyroid hormone and AAV-Atoh1 sets of experiments. The simplest explanation for this observation is that genetic drift has caused HEI-OC1 cells to lose prestin expression over the numerous generations that have passed since their development. In fact, drift has previously been reported for some HEI-OC1 populations in regard to aminoglycoside sensitivity20,21. While CR-OSCs are not exempt from genetic drift, e.g. after 5 generations of progressively passaging CR-OSCs, there was a small but significant reduction in the expression levels of myosin VIIa and myosin VI, this genetic drift can be minimized by generating CR-OSCs on demand from live mice and keeping the passage numbers below 10 Our observations therefore not only highlight the impact that genetic drift can have on various cell lines and the need to constantly verify the integrity of the cell line being utilized, but suggest an advantage of the CR-OSC technique in the ability of every investigator to produce these cells in their own laboratory and not rely on cell line banks or other investigators for their cells.
Another advantage of the CR-OSC method that is of great importance is that CR-OSCs can be derived from any mouse model available, opening up vast resources of genetically modified mouse strains to use in conjunction with CR-OSCs. Thus, cell lines with fluorescent or luminescent reporters can be easily derived for reporter expression analysis in response to pharmacologic or genetic manipulation. Similarly, experiments involving genetic mutations, knock-outs, or overexpression can be tested on large numbers of cochlear cells with relative ease. This is particularly useful in cases where crosses of mice carrying multiple transgenic alleles yield small numbers of double, triple, or quadruple positive mutants, or in cases where the desired genetic manipulations are lethal at embryonic or perinatal ages. Furthermore, the large numbers of cells derived from each cochlea provides enhanced investigative capability in probing the molecular mechanisms or consequences of genetic manipulations. For example fluorescence activated cell sorting (FACS) and whole genome (DNA-seq or ChIP-seq), whole transcriptome (RNA-seq), or whole proteome (mass spectrometry) experiments are less costly and more expedient when large numbers of cells are available for use. In this way, investigators can garner large datasets resulting from a given genetic manipulation or from the enrichment of a cell population that expresses a fluorescent reporter without having to breed, euthanize and dissect and pool cochleae from large numbers of mice simultaneously.
Similarly, CR-OSCs are readily amenable for use in high throughput screening strategies. Even after being passaged to generated sufficiently large numbers of cells, CR-OSCs retain the ability to respond to manipulations that are known to promote a HC fate (Atoh1 overexpression29,30,31,32), or regulate prestin expression (Thyroid hormone9,10,11). CR-OSCs responded to both treatments as expected, upregulating early HC markers in response to Atoh1 overexpression and upregulating prestin expression in response to thyroid hormone treatment. These results together indicate that CR-OSCs could readily be purposed toward high throughput screens for small molecules or genetic factors that promote the expression of HC-specific genes, including prestin and that ectopic Atoh1 expression or the application of thyroid hormones could be used as positive control conditions for assay development.
CR-OSCs could potentially be utilized for questions regarding IHC vs OHC fate, as differentiation conditions upregulated the OHC-specific gene prestin4,5,38 while having no effect on the IHC-enriched genes otoferlin39,40 and VGlut341,42. Alternatively, VGlut3 and otoferlin are both synaptic proteins and may not be upregulated because of the lack of synaptic connections in our conditions. If this scenario is correct, one could hypothesize that co-culturing CR-OSCs with neurons during differentiation may result in the up-regulation of these genes, a hypothesis that would require further testing in future experiments.
Similarly, our AAV transduction experiments provided some unexpected insights. While it is well known that in vivo Atoh1 transduction can promote immature HC phenotypes, the up-regulation of otoferlin was unexpected. These data suggest that the gene networks modulated by Atoh1 overexpression may be involved in signaling pathways essential for cell fate decisions to produce IHCs, which is consistent with ideas put forth by Jahan et al.43. Reports have also shown that otoferlin is highly expressed in vestibular hair cells44 and that Atoh1 overexpression may select for vestibular type hair cells when overexpressed in the cochlea45. Thus, our data may also be supportive of the idea that Atoh1 overexpression may selectively up-regulate gene targets that are enriched in both vestibular and inner hair cells. Consistent with this notion, AAV mediated transduction of Atoh1 in CR-OSCs did not result in up-regulation of the OHC-specific gene prestin. We were also struck by the lack of response observed in the HEI-OC1 cells following Atoh1 overexpression. This observation may be due to the lower transduction efficiency displayed by the HEI-OC1 cells (100 fold increase vs. 1000 fold increase in CR-OSCs), but this may also portend the inflexibility of the transcriptional networks inherent to the HEI-OC1 cells. It is also possible that the permissive (proliferative) conditions used in our studies did not allow for the proper up-regulation of normal transcriptional networks. However, the original characterization of the HEI-OC1 cells reported that HEI-OC1 cells express all assayed HC markers in both permissive and non-permissive conditions14.
Finally, CR-OSCs may offer a new method for studying otic progenitor cells, specifically with regard to why these cells lose their cellular plasticity during early postnatal development. These data lend evidence that it may be possible to dissect out the factors that lead to the pseudo-immortalization caused by the CR conditions. Indeed, the results presented here suggest that the activities of telomerase and Rho-associated coiled-coil containing protein kinases are likely involved in the senescence of the otic stem cell population. Though, further testing will be required to see if these factors exert similar influence in vivo, as well as to determine the necessary factors that are being provided by the feeder cells. Once these factors are better elucidated, researchers may be able to manipulate conditions in vivo to help maintain the cellular plasticity that is normally lost in cochlea during postnatal development.
Although CR-OSCs have important implications for understanding hair cell development and the transcriptional regulation of several HC-specific gene products, further characterization of HC bundle formation, electrophysiological responses and the translation of HC-specific genes into proteins will be required to determine the extent to which these cells have the potential to give rise to functional HCs. Importantly, only about five percent of the CR-OSCs expressed Myo6 and in a portion of this cell population, we observed Prestin expression. On one hand, it reflects the heterogeneity of CR-OSCs similar to many other stem/progenitor cell lines (e.g. HEI-OCs). On the other hand, the nature of the remaining cells remains elusive. A reasonable hypothesis is that they are supporting cell-like because more than 80% of differentiated otic spheres cultured without CR expressed the supporting cell markers pan-Cytokeratin22 and Sox246. Here, we attempted to immunostain the differentiated CR-OSCs with antibodies recognizing the supporting cell markers Sox2 and Prox1, as well as the neuronal markers Tuj1 and NeuN and the mesenchymal cell marker Axin2. However, we detected no convincing signal for these proteins. One possibility is that the immunocytochemistry technique as applied here was not sensitive enough to detect these proteins, although all of the antibodies used did produce positive signal in vivo. The other possibility is that the CR condition inhibited the differentiation from otic progenitor cells to supporting cells, or promoted de-differentiation away from the otic lineage, perhaps even to more stem-like cells. Given the interesting and elusive nature of these cells, our future studies are aimed at characterizing this cell population.
In summary, we have described the first otic progenitor cell line capable of producing large quantities of cells that can transcriptionally regulate both early and terminal HC genes and retain the ability to respond normally to cues designed to promote HC fate. However, one of the most valuable aspects of the CR-OSCs is the ability to generate them in any laboratory as needed and to utilize any genetically modified mouse model. This study provides inner ear researchers a completely novel in vitro tool for the study of hair cell development and/or responses to pharmacologic or genetic manipulation and offers the field a primary otic progenitor cell line for high throughput screening strategies aimed at ways to promote the expression of HC-specific genes and possibly even terminal hair cell differentiation.
Additional Information
How to cite this article: Walters, B. J. et al. Pseudo-immortalization of postnatal cochlear progenitor cells yields a scalable cell line capable of transcriptionally regulating mature hair cell genes. Sci. Rep. 5, 17792; doi: 10.1038/srep17792 (2015).
Change history
25 February 2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Willott, J. F. Handbook of mouse auditory research: from behavior to molecular biology. (CRC Press, 2001).
Dallos, P. & Evans, B. N. High-frequency motility of outer hair cells and the cochlear amplifier. Science 267, 2006–2009 (1995).
Dallos, P. et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58, 333–339 (2008).
Liberman, M. C. et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419, 300–304 (2002).
Belyantseva, I. A., Adler, H. J., Curi, R., Frolenkov, G. I. & Kachar, B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci 20, RC116 (2000).
Griffith, A. J. et al. Knock-in mouse model for resistance to thyroid hormone (RTH): an RTH mutation in the thyroid hormone receptor beta gene disrupts cochlear morphogenesis. J Assoc Res Otolaryngol 3, 279–288 (2002).
Rusch, A. et al. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci 21, 9792–9800 (2001).
Richter, C. P., Munscher, A., Machado, D. S., Wondisford, F. E. & Ortiga-Carvalho, T. M. Complete activation of thyroid hormone receptor beta by T3 is essential for normal cochlear function and morphology in mice. Cell Physiol Biochem 28, 997–1008 (2011).
Winter, H. et al. Thyroid hormone receptors TRalpha1 and TRbeta differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci 119, 2975–2984 (2006).
Weber, T. et al. Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci USA 99, 2901–2906 (2002).
Gross, J., Angerstein, M., Fuchs, J., Stute, K. & Mazurek, B. Expression analysis of prestin and selected transcription factors in newborn rats. Cell Mol Neurobiol 31, 1089–1101 (2011).
Holley, M. C., Nishida, Y. & Grix, N. Conditional immortalization of hair cells from the inner ear. Int J Dev Neurosci 15, 541–552 (1997).
Holley, M. C. & Lawlor, P. W. Production of conditionally immortalised cell lines from a transgenic mouse. Audiol Neurootol 2, 25–35 (1997).
Kalinec, G. M., Webster, P., Lim, D. J. & Kalinec, F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol 8, 177–189 (2003).
Germiller, J. A. et al. Molecular characterization of conditionally immortalized cell lines derived from mouse early embryonic inner ear. Dev Dyn 231, 815–827 (2004).
Jagger, D. J., Holley, M. C. & Ashmore, J. F. Ionic currents expressed in a cell line derived from the organ of Corti of the Immortomouse. Pflugers Arch 438, 8–14 (1999).
Salehi, P. et al. Attenuation of Cisplatin Ototoxicity by Otoprotective Effects of Nanoencapsulated Curcumin and Dexamethasone in a Guinea Pig Model. Otol Neurotol (2014).
Shin, Y. S. et al. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: In vitro and in vivo study. Neuroscience 232C, 1–12 (2012).
More, S. S. et al. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci 30, 9500–9509 (2010).
Chen, F. Q., Hill, K., Guan, Y. J., Schacht, J. & Sha, S. H. Activation of apoptotic pathways in the absence of cell death in an inner-ear immortomouse cell line. Hear Res 284, 33–41 (2012).
Cederroth, C. R. Loss of aminoglycoside sensitivity in HEI-OC1 cells ? Hear Res 292, 83–85, author response pg 86 (2012).
Diensthuber, M., Oshima, K. & Heller, S. Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J Assoc Res Otolaryngol 10, 173–190 (2009).
Yerukhimovich, M. V., Bai, L., Chen, D. H., Miller, R. H. & Alagramam, K. N. Identification and characterization of mouse cochlear stem cells. Dev Neurosci 29, 251–260 (2007).
Wang, Z. et al. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport 17, 767–771 (2006).
Oshima, K. et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol 8, 18–31 (2007).
Liu, X. et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol 180, 599–607 (2012).
Saenz, F. R. et al. Conditionally reprogrammed normal and transformed mouse mammary epithelial cells display a progenitor-cell-like phenotype. PLoS One 9, e97666 (2014).
Suprynowicz, F. A. et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA 109, 20035–20040 (2012).
Liu, Z., Fang, J., Dearman, J., Zhang, L. & Zuo, J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One 9, e89377 (2014).
Yang, J., Cong, N., Han, Z., Huang, Y. & Chi, F. Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci Lett 549, 7–11 (2013).
Liu, Z. et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci 32, 6600–6610 (2012).
Woods, C., Montcouquiol, M. & Kelley, M. W. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 7, 1310–1318 (2004).
Zheng, J. L. & Gao, W. Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci 3, 580–586 (2000).
Kawamoto, K., Ishimoto, S., Minoda, R., Brough, D. E. & Raphael, Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 23, 4395–4400 (2003).
Fang, J. et al. Outer hair cell-specific prestin-CreERT2 knockin mouse lines. Genesis 50, 124–131 (2012).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140 (2010).
Kelly, M. C., Chang, Q., Pan, A., Lin, X. & Chen, P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci 32, 6699–6710 (2012).
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000).
Yasunaga, S. et al. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am J Hum Genet 67, 591–600 (2000).
Yasunaga, S. et al. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet 21, 363–369 (1999).
Ruel, J. et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet 83, 278–292 (2008).
Seal, R. P. et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 57, 263–275 (2008).
Jahan, I., Pan, N., Kersigo, J. & Fritzsch, B. Beyond generalized hair cells: molecular cues for hair cell types. Hear Res 297, 30–41 (2013).
Goodyear, R. J. et al. Identification of the hair cell soma-1 antigen, HCS-1, as otoferlin. J Assoc Res Otolaryngol 11, 573–586 (2010).
Yang, J., Bouvron, S., Lv, P., Chi, F. & Yamoah, E. N. Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. J Neurosci 32, 3712–3725 (2012).
Shi, F., Kempfle, J. S. & Edge, A. S. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci 32, 9639–9648 (2012).
Acknowledgements
We thank Dr. Richard Schlegel for providing the 3T3-J2 cells, Dr. Kalenic and the House Research Institute for providing the HEI-OC1 cells, Dr. Guillermo Oliver for the MEFs, Dr. Suzanne Baker for the NIH 3T3 cells and Drs. Stefan Heller and Kazuo Oshima for help on isolation of otic-spheres and Dr. Yannan Ouyang for help on confocal microscope imaging.
Author information
Authors and Affiliations
Contributions
Brandon J. Walters, S.D., F.Z., Bradley J. Walters and W.S.L. designed the experiment(s), Brandon J. Walters, S.D. and F.Z. conducted the experiment(s), Brandon J. Walters, S.D., F.Z. and J.Z. analyzed the results. Brandon J. Walters, F.Z., Bradley J. Walters and W.S.L. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Walters, B., Diao, S., Zheng, F. et al. Pseudo-immortalization of postnatal cochlear progenitor cells yields a scalable cell line capable of transcriptionally regulating mature hair cell genes. Sci Rep 5, 17792 (2015). https://doi.org/10.1038/srep17792
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17792
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.